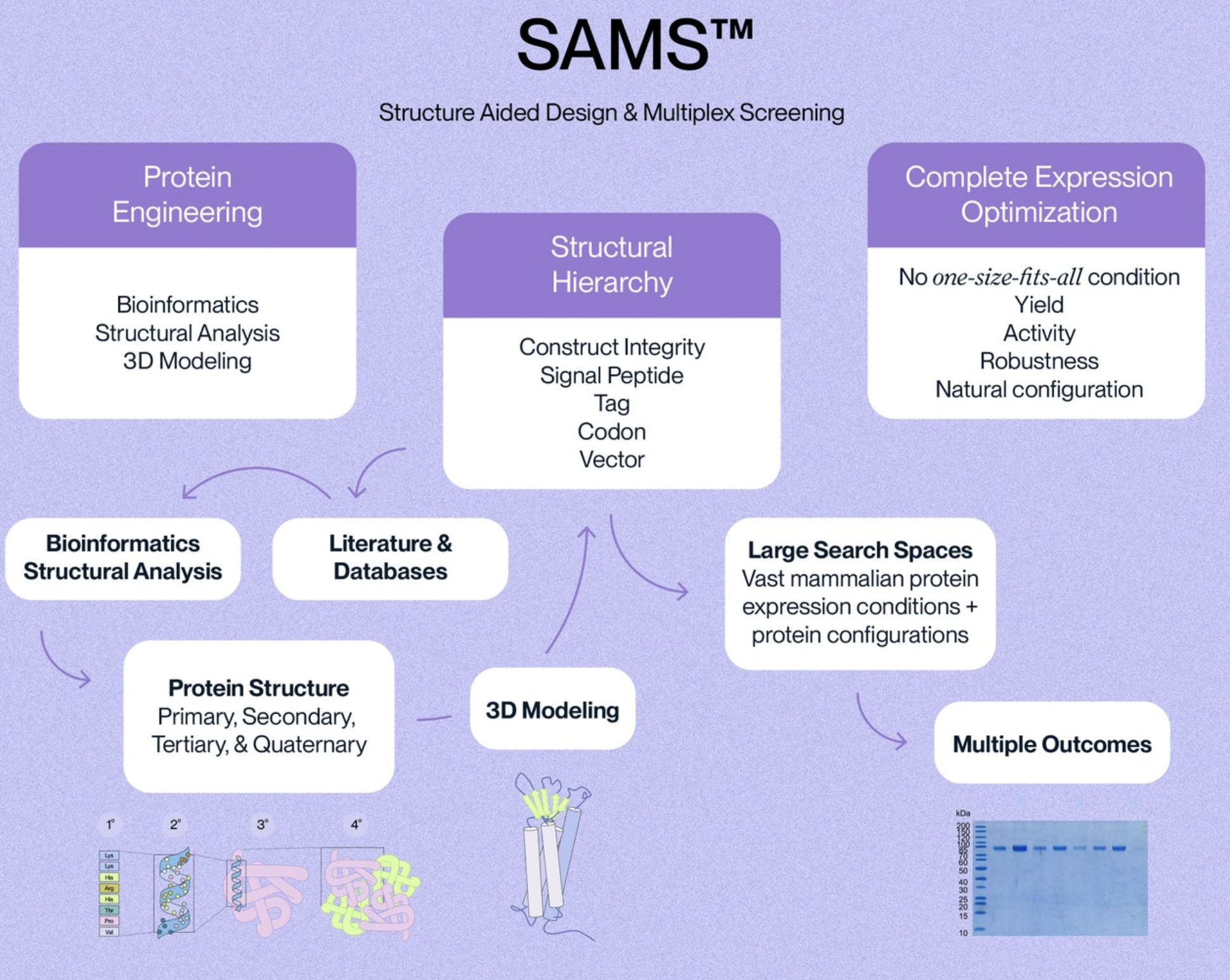
Quality control is a critical aspect of recombinant protein production that ensures the purity, potency, and consistency of the final product. Our quality control systems are designed to monitor and assess the quality of the production process and final product.
Process Validation
We monitor the entire production process to ensure expression conditions are optimal for that specific protein. This includes monitoring the growth of the host cells, expression of the target gene, and purification of the recombinant protein. The expression process is optimized to minimize the presence of contaminants that can affect the purity or activity of the final product.
Analytical Testing
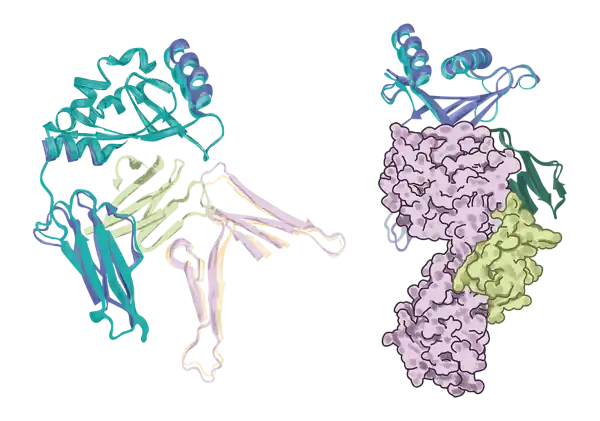
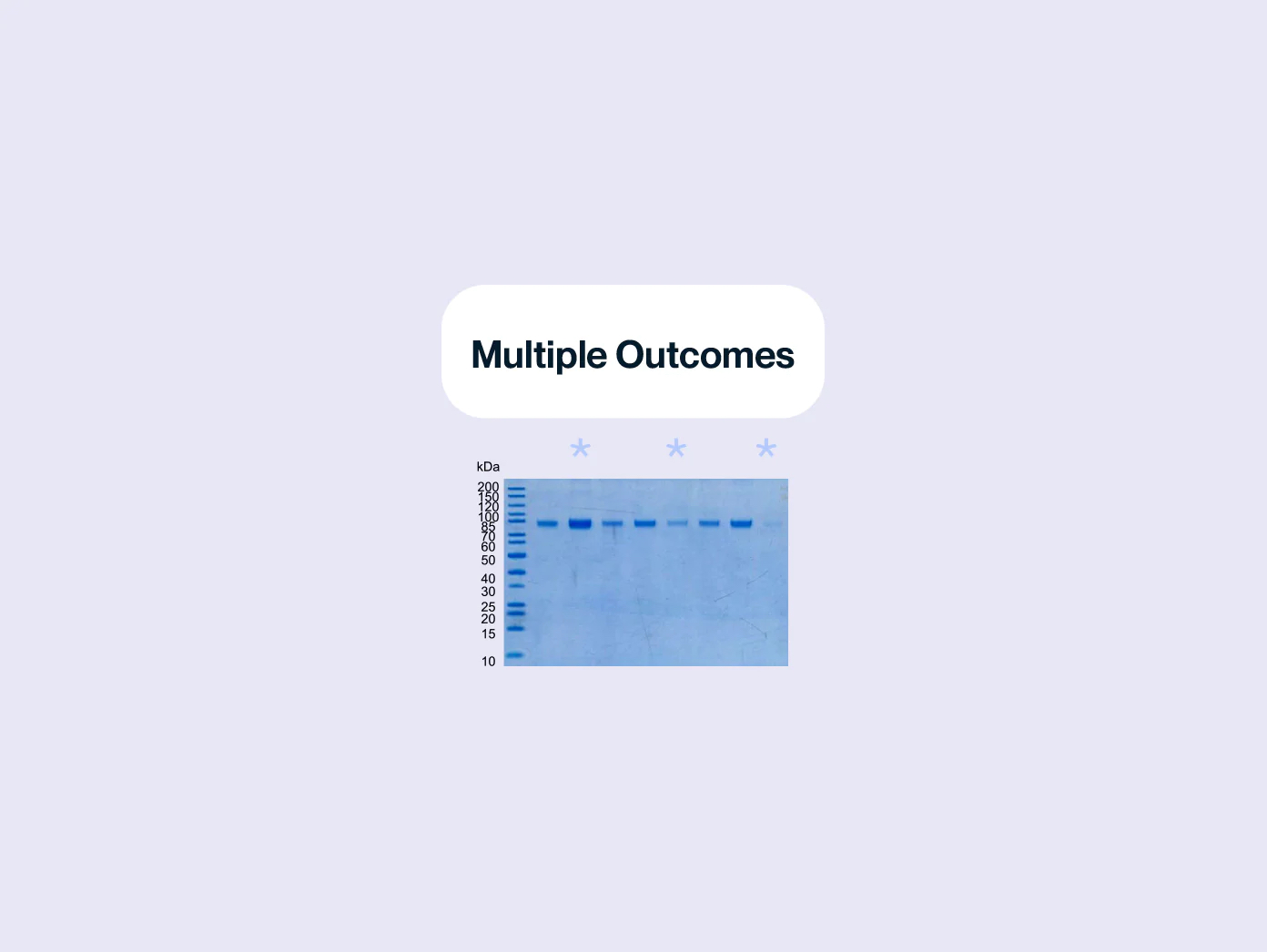
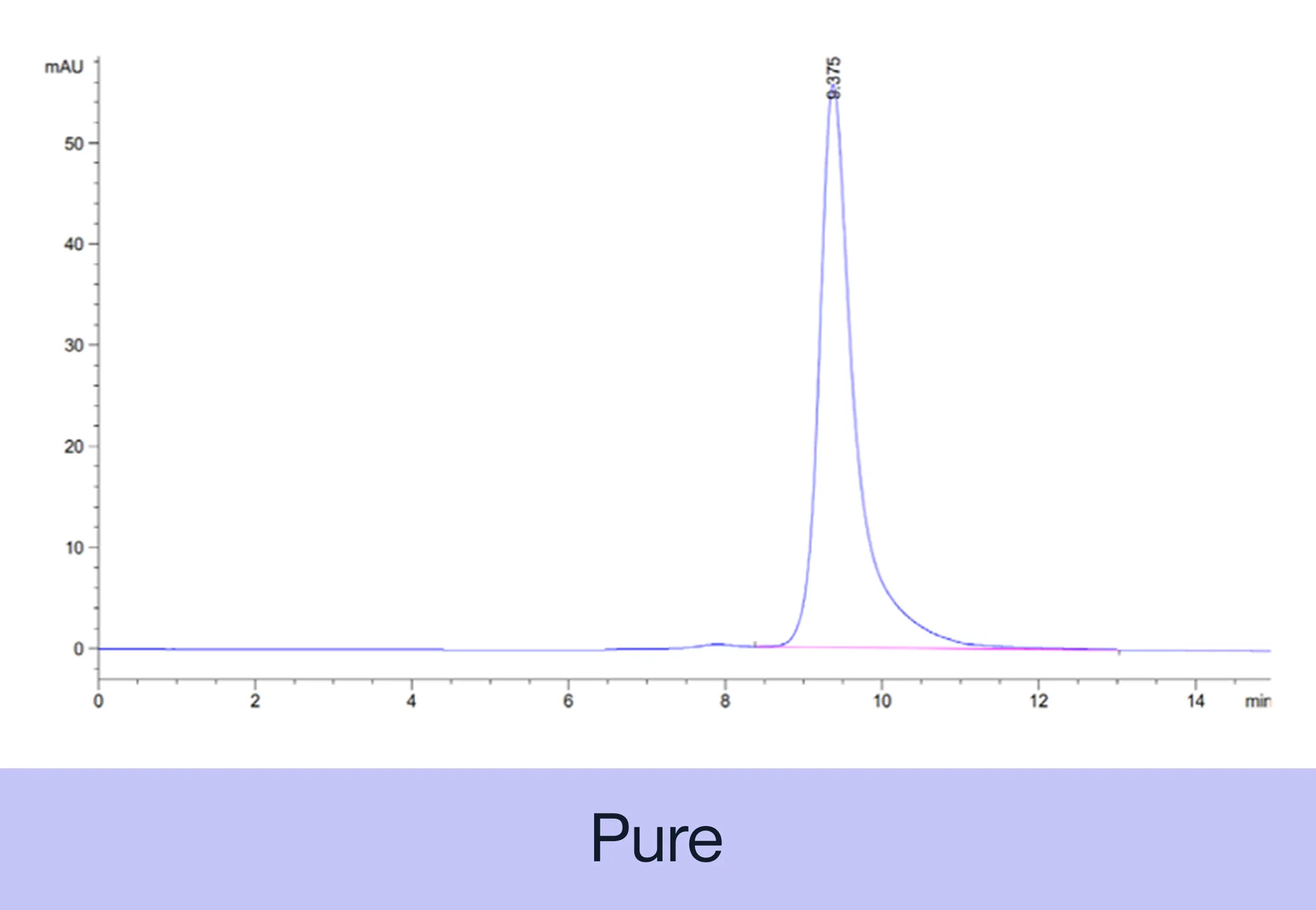
PURITY The purity of each recombinant protein is ensured using HPLC and by quantifying the level of impurities, such as host cell proteins, endotoxin, and host cell DNA in the final product.
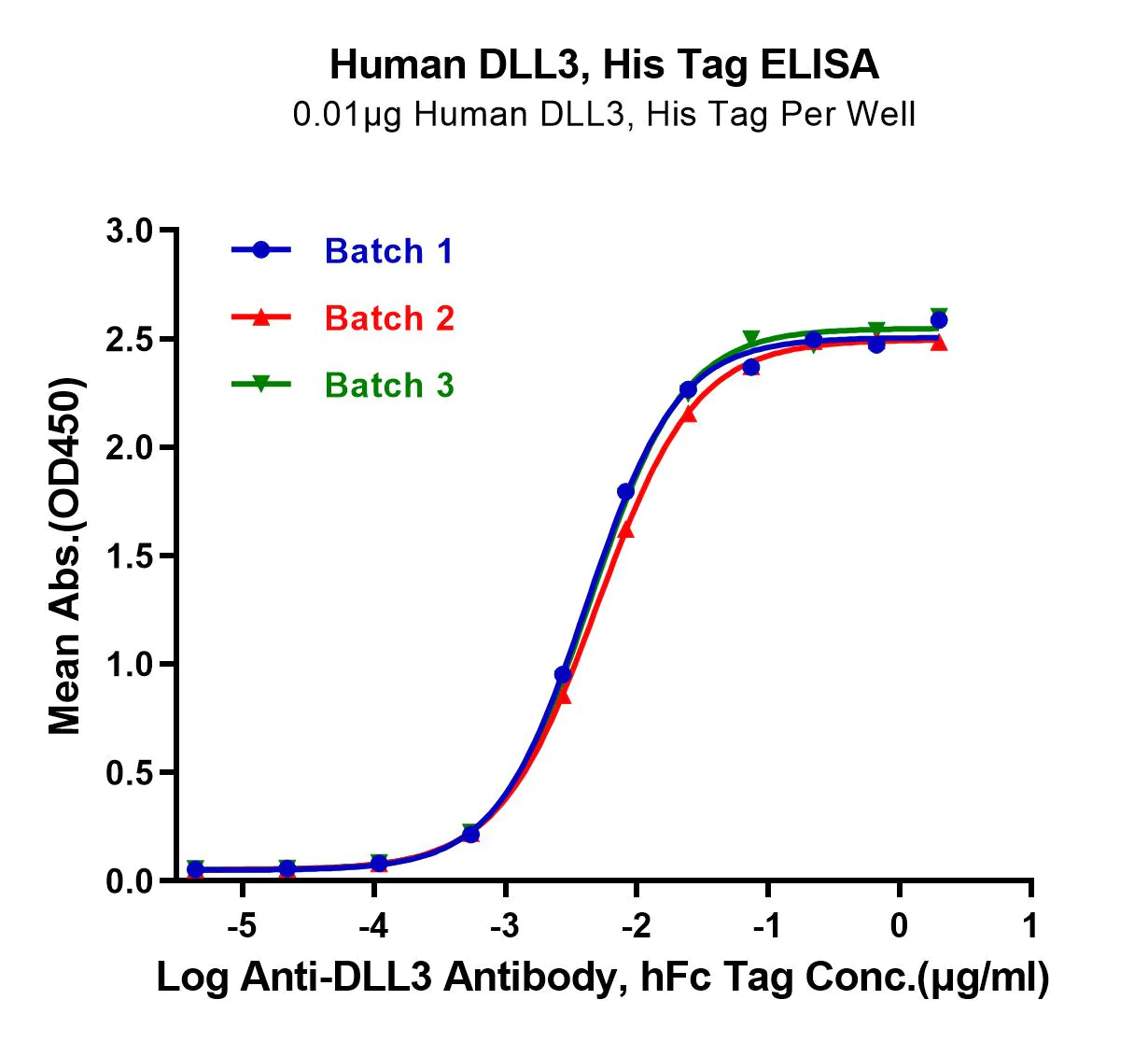
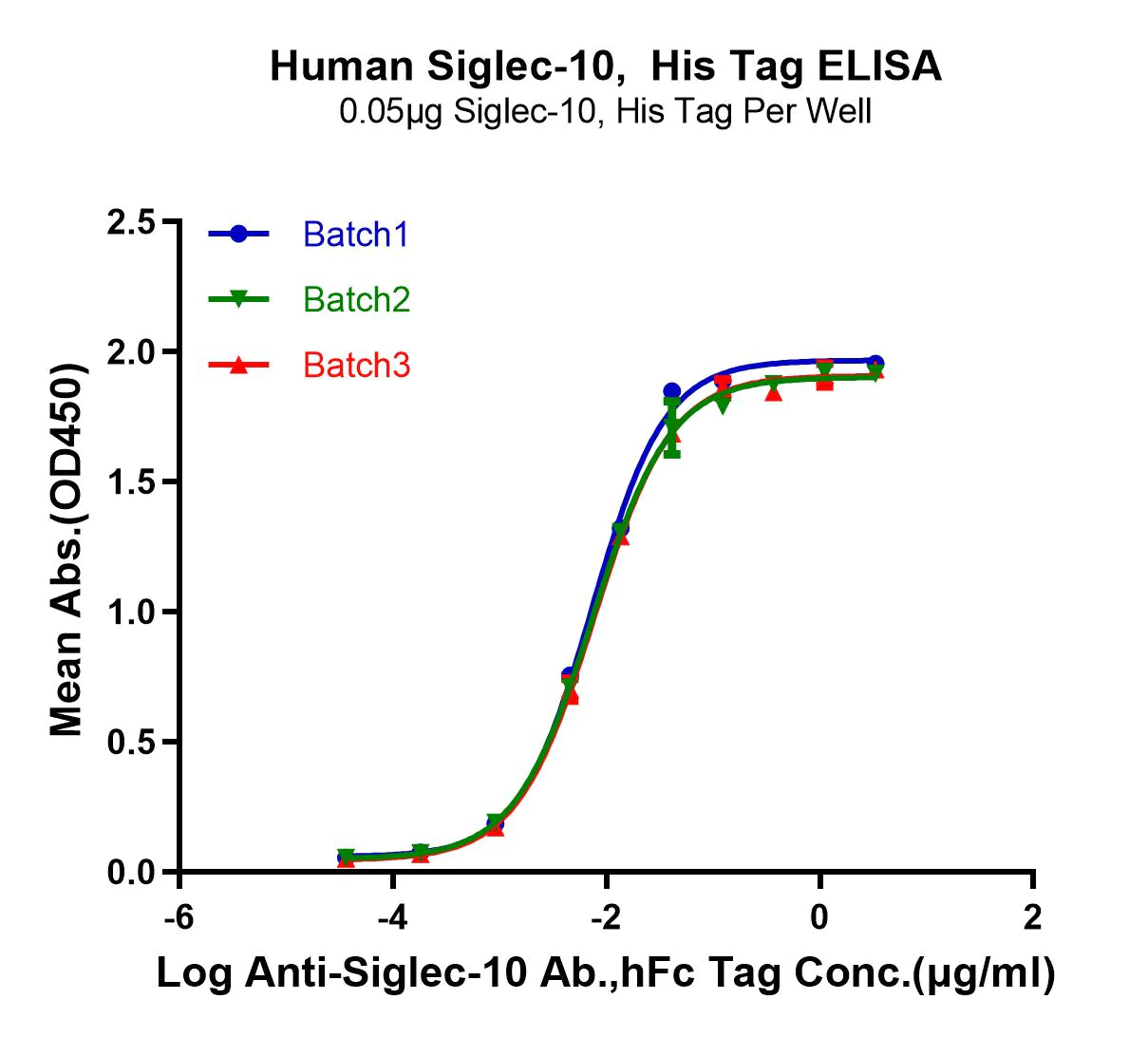
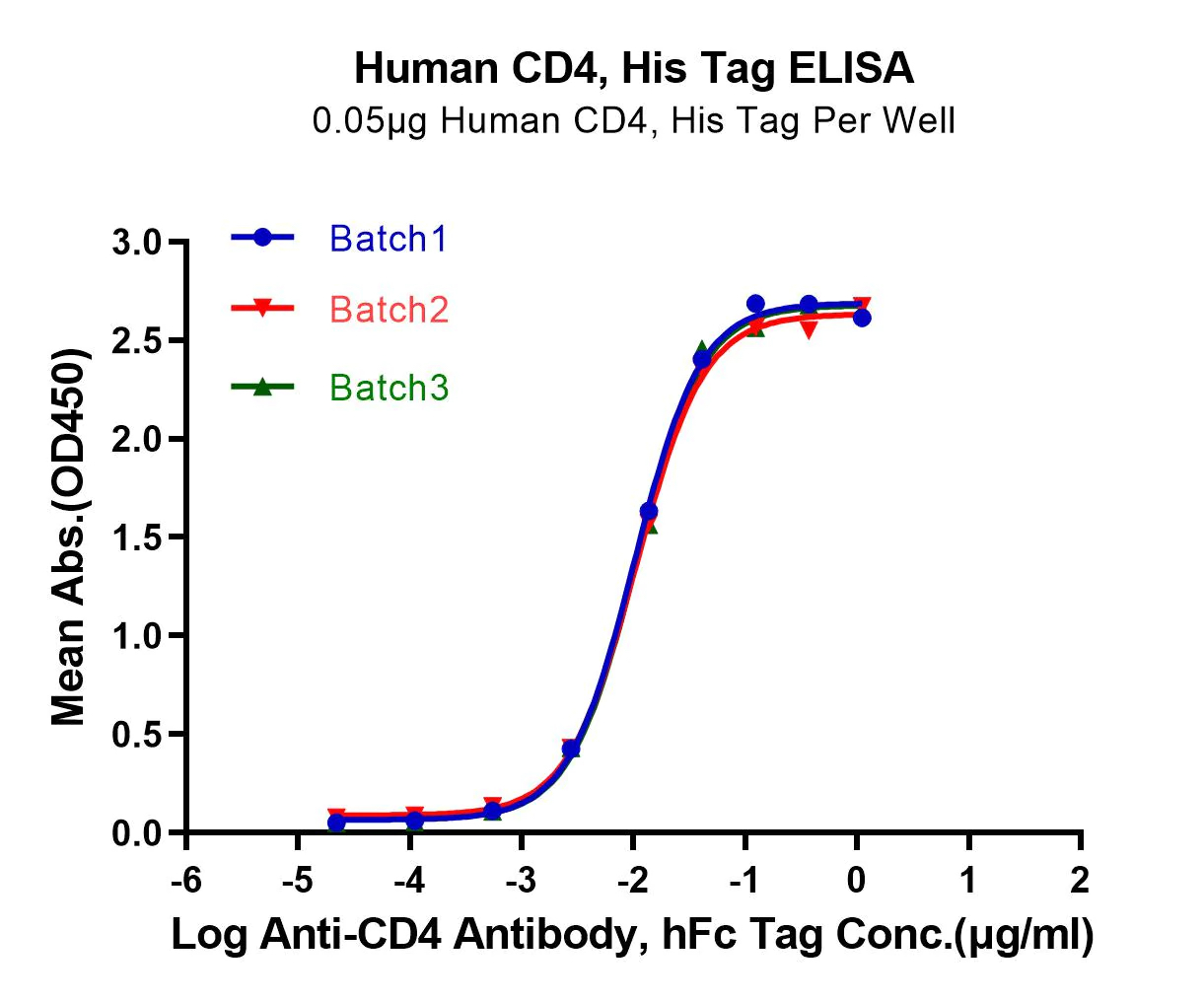
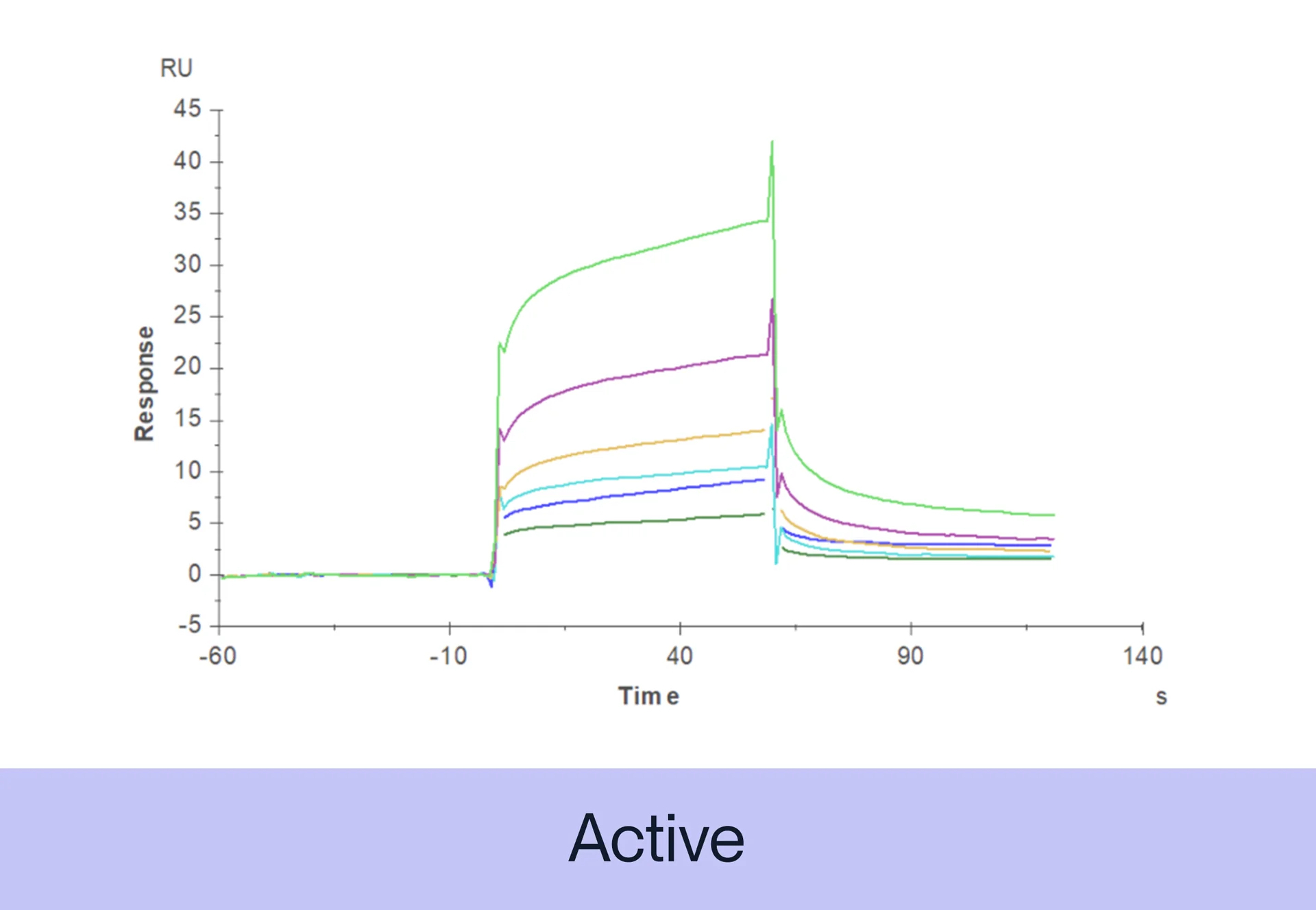
ACTIVITY The potency of our recombinant proteins is analyzed by measuring its in vitro activity, such as its ability to bind to its target molecule via ELISA or SPR assay.
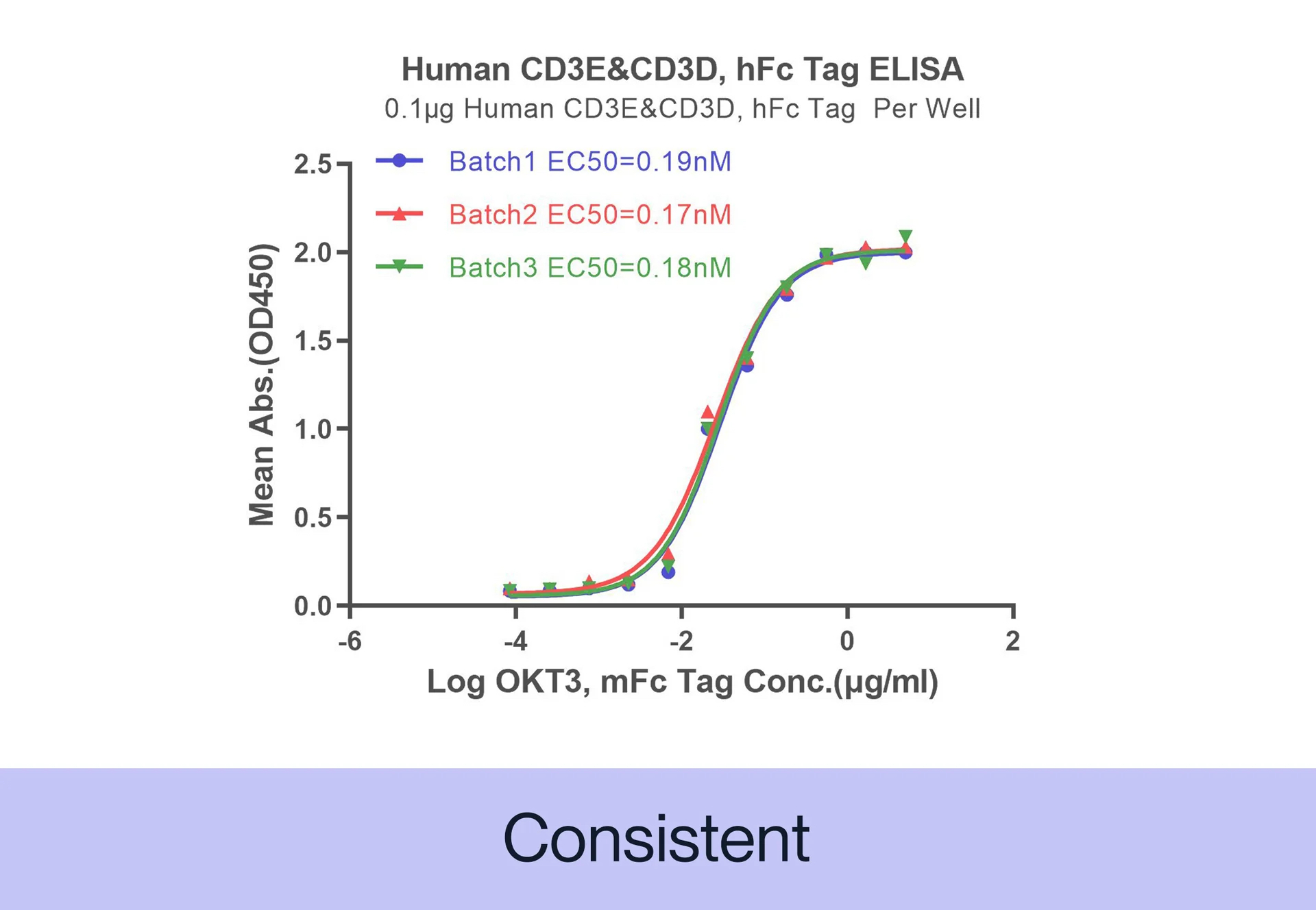
CONSISTENCY Our products are tested for batch-to-batch consistency to ensure stable bioactivity across lots. Additionally, we assess stability of our products by measuring degradation over time and resistance to environmental stress factors, such as temperature and pH changes.