Creative Diagnostics staff scientists have extensive antibody purification experience. We have developed robust proprietary purification procedures for antibodies from serum, ascites fluid, yolks and culture supernatants for use in early discovery research, preclinical trials and in vitro medical devices. All purified antibodies can be analyzed by electrophoresis, ELISA, western blot and HPLC to determine purity and integrity. Corresponding test data will be provided to the customer.
Available antibody purification services and methods include:
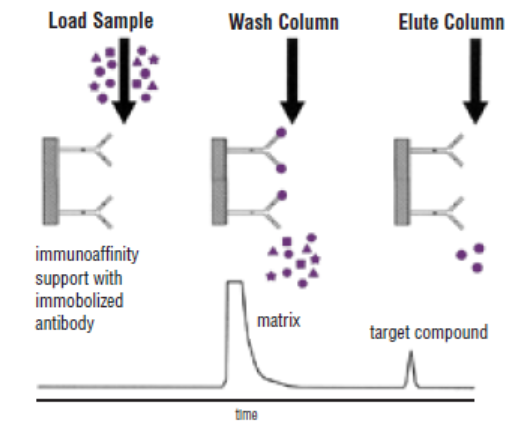
Antigen/Antibody Affinity Purification
Antigen/Antibody affinity can be used to obtain extremely pure specific antibodies from complex samples. Due to the highly specific purification process, the resulting antibody preparation tends to have lower background levels and lower non-specific binding rates. Our scientists have generated various affinity columns, like target-specific peptides, recombinant proteins and antibody-based column, and employed several key procedures to provide the best possible affinity-purified antibodies.

Protein A and Protein G Affinity Chromatography
Protein A and Protein G are the most common choices for antibody purification due to their ability to bind the constant (Fc) region of IgG from various species. Since Protein A and Protein G have differing binding efficiencies for IgG from different species, it is important to check this before deciding which method to use. Protein A/G, a recombinant fusion protein that combines IgG binding domains of both Protein A and Protein G, has the additive properties of Protein A and G.
| ANTIBODY SOURCE |
RECOMMENDED |
| Mouse ascites fluid |
Protein G |
| Rat ascites fluid |
Protein G |
| Mouse bioreactor tissue culture supernatant |
Protein G |
| Rabbit monoclonal |
Protein G |
| Raw rabbit serum |
Protein A |
| Raw goat or sheep sera |
Protein A |
Protein L Affinity Chromatography
Protein L binds antibodies through kappa light chain interactions. Protein L binds a wider range of antibody classes than Protein A or G, including ScFv and Fab fragments. Protein L is extremely useful for purification of antibodies from culture supernatant because it does not bind bovine immunoglobulins, which are often present in the media as a serum supplement. Also, Protein L does not interfere with the antigen-binding site of the antibody, making it useful for immunoprecipitation assays, even using IgM.
IgY Purification from Egg Yolk
Chickens produce unique antibodies called IgY. Our team has developed multi-step purification, concerning PEG precipitation, ion exchange and Antigen/ Protein L affinity chromatography, to purify and enrich specific IgY antibodies from chicken egg yolk. Typically, we need to test the starting material by ELSIA assay and run SDS-PAGE to analysis the final purified antibodies.
Aseptic/Low Endotoxin Purification
Creative Diagnostics understand that each customer has unique requirements for their project. That’s why we are completely flexible with custom purification for list antibody products. We can custom provide 0.2 micron sterile-filtered, Low Endotoxin and Azide-Free antibodies for in vivo and in vitro assays. We are here to meet your needs.
Creative Diagnostics understand that each customer has unique requirements for their project. That’s why we are completely flexible with custom purification for list antibody products. We can custom provide 0.2 micron sterile-filtered, Low Endotoxin and Azide-Free antibodies for in vivo and in vitro assays. We are also committed to provide the highest quality and standards.
Functional antibodies can either mimic or interrupt the natural biologic effects associated with ligand-receptor interactions, or have a physiological effect on a target cell or molecule. They may be used in several functional assays, including depletion, activation, neutralization, or blocking experiments, both in vitro and in vivo. Functional grade antibodies are available free of preservatives and tested for low endotoxin content.
Low endotoxin purification
Endotoxins, toxic substance bound to the bacterial cell wall and released when the bacterium ruptures or disintegrates. Removal of endotoxin is one of the most difficult downstream processes during antibody purification, because endotoxin is extremely heat and pH stable. Our team has developed a low endotoxin purification process that serves to minimize endotoxin loads. Besides, our rapid test methods allow us to produce results right at the point of antibody purification.
Sodium azide removal
Sodium azide is a preservative used for inhibiting the growth of contaminants, such as bacteria or fungi. However, its presence in antibody solutions can affect the use of the antibody in cell culture assays as it is toxic to cells. It can also interfere with antibody conjugation and inhibits the activity of the enzyme horseradish peroxidase. Many CD antibody products contain sodium azide and this information is provided on individual datasheets. If the antibody is to be used for cell culture assays or conjugation, sodium azide removal from the antibody solution is recommended. Creative Diagnostics can provide sodium azide removal service to meet customer’s needs.
The following three procedures can be used to remove azide:
1. Dialysis
2. Desalting
3. Antibody purification kit
Custom Options
- Bulk production from your hybridoma.
- Custom purification from our list antibodies.
- Custom concentrations available.
- Custom endotoxin limits.
- Flexible packaging.
Additional Service:
We also offer following services, which could be utilized dependently or independently, to support our customs’ antibody purification program.
- HPLC purification
- Size Exclusion/ Gel Filtration Chromatography
- Ion Exchange
- Hydrophobic Interaction
How it Works:
Working with Creative Diagnostics on antibody purification is easy: simply let us know some basic information, including:
- Type of purification wanted
- Protein being purified (ex: size, storage temperature, pH, toxicity)
One of our specialists will be in touch to confirm all of the information. Once the quote is approved, simply send in the protein to our laboratory or use our listed antibody products and within 2-3 weeks, the purified antibody will be sent to your lab.


.jpg)
.jpg)
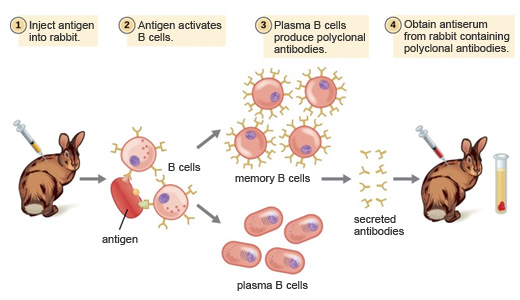
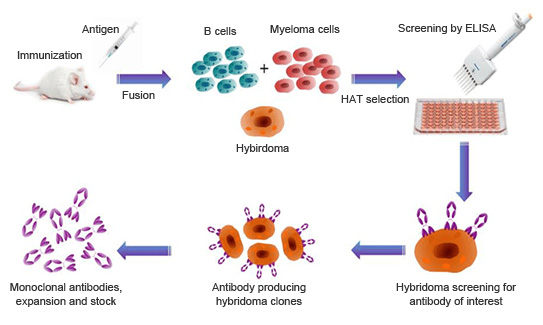
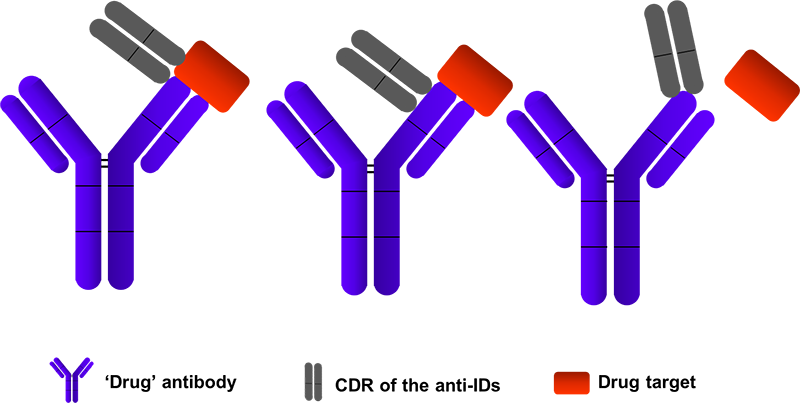

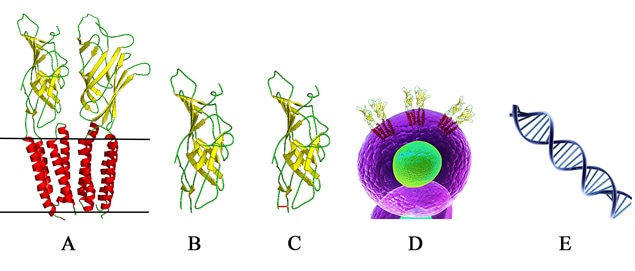
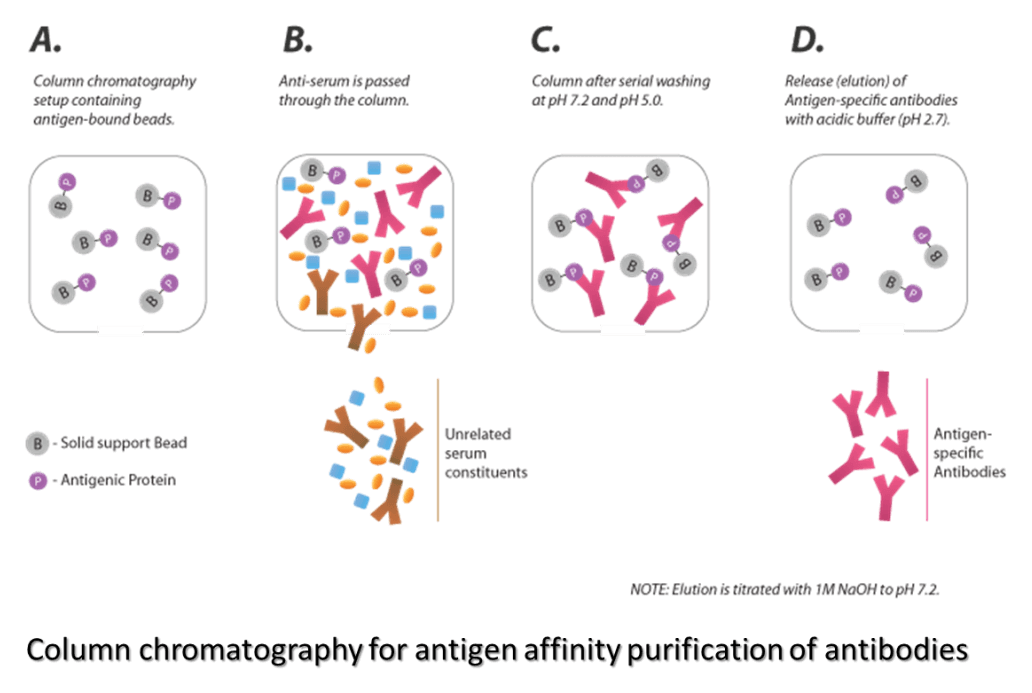
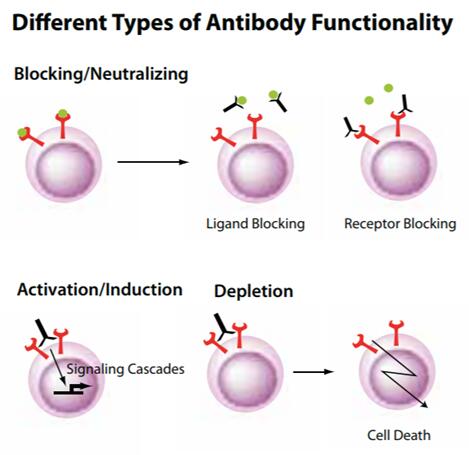

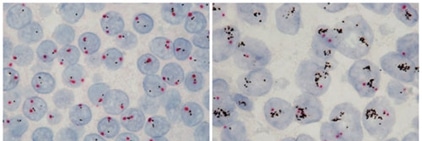
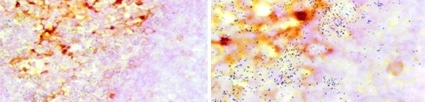
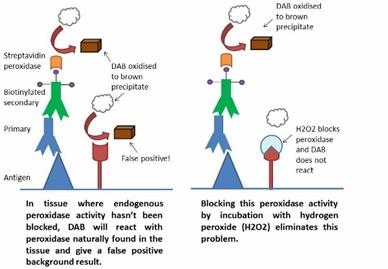
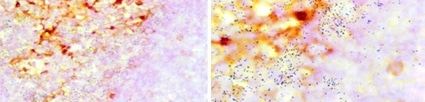
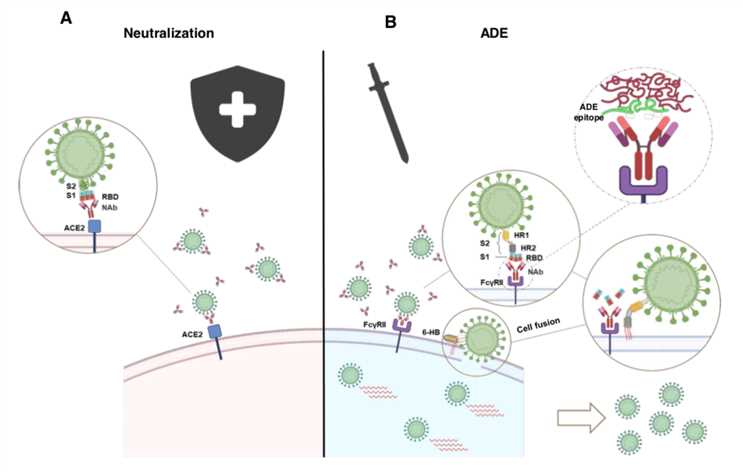 Figure 1: ADE activity generation. Adapted from: Wu, F., Yan, R., Liu, M., Liu, Z., Wang, Y., Luan, D., … & Huang, J. (2020). Antibody-dependent enhancement (ADE) of SARS-CoV-2 infection in recovered COVID-19 patients: Studies based on cellular and structural biology analysis. medRxiv.
Figure 1: ADE activity generation. Adapted from: Wu, F., Yan, R., Liu, M., Liu, Z., Wang, Y., Luan, D., … & Huang, J. (2020). Antibody-dependent enhancement (ADE) of SARS-CoV-2 infection in recovered COVID-19 patients: Studies based on cellular and structural biology analysis. medRxiv.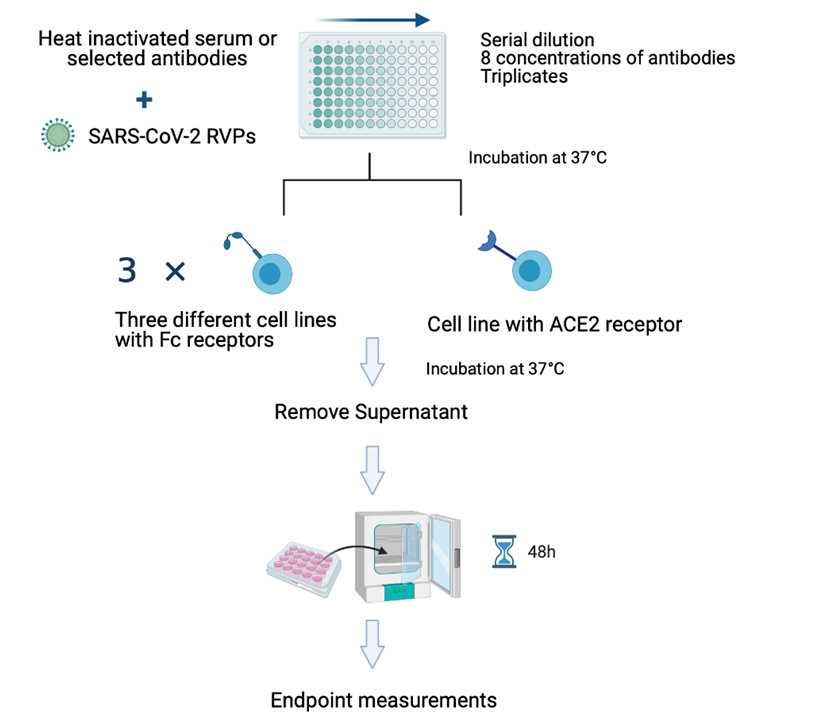
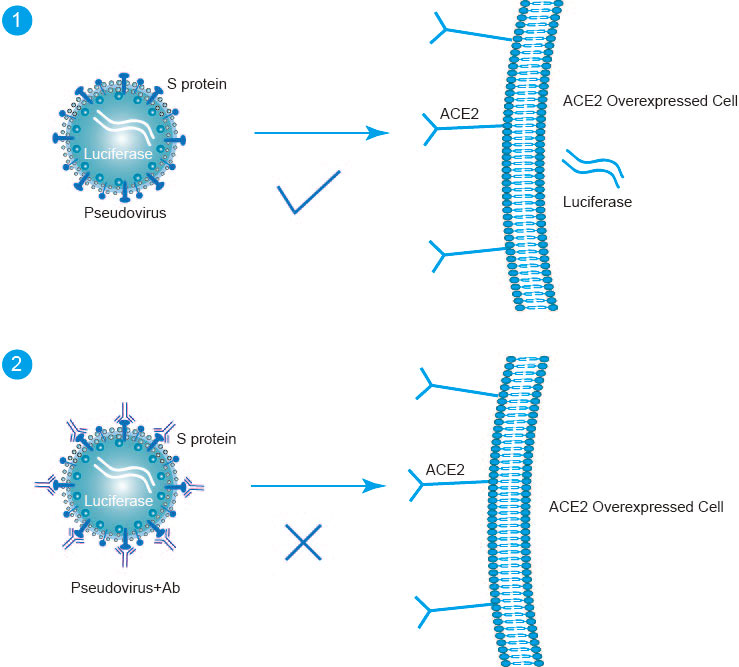 Fig1. Pseudotype-Based Neutralization Assays Principle
Fig1. Pseudotype-Based Neutralization Assays Principle