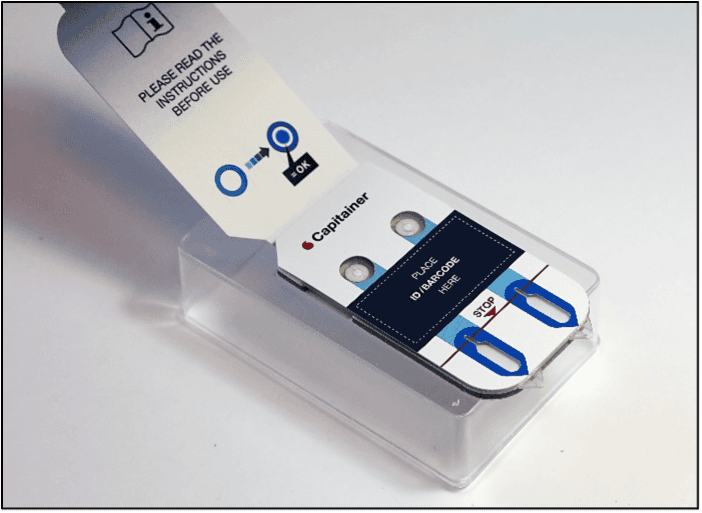
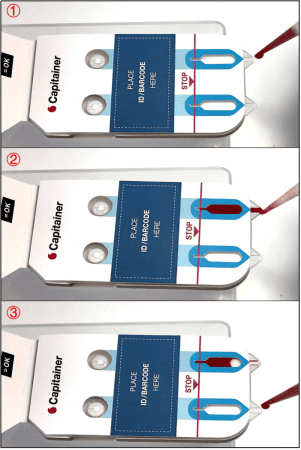
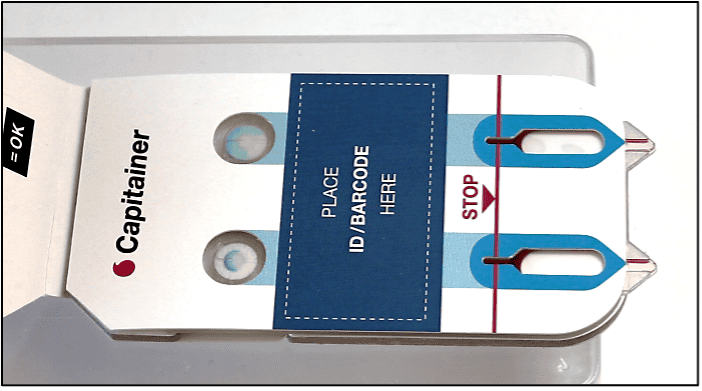
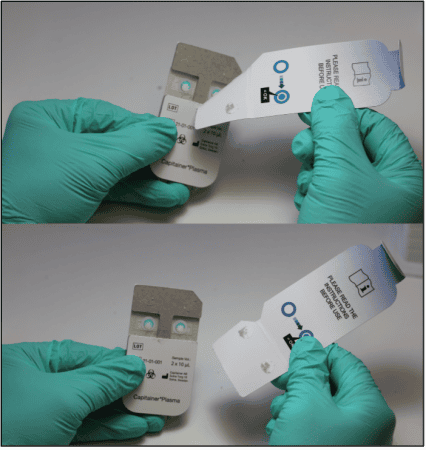
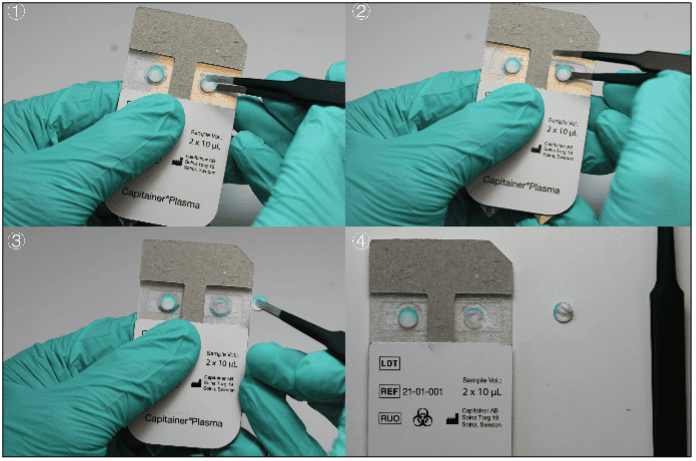
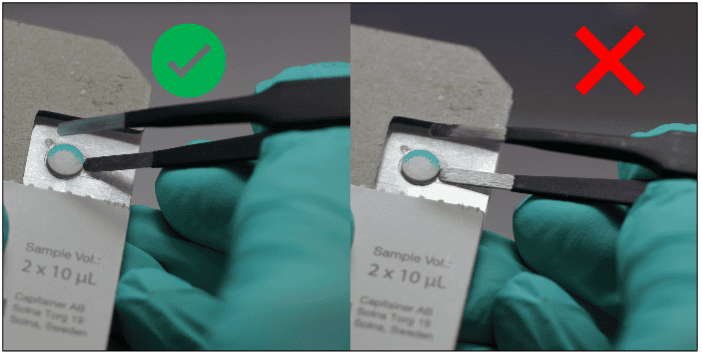
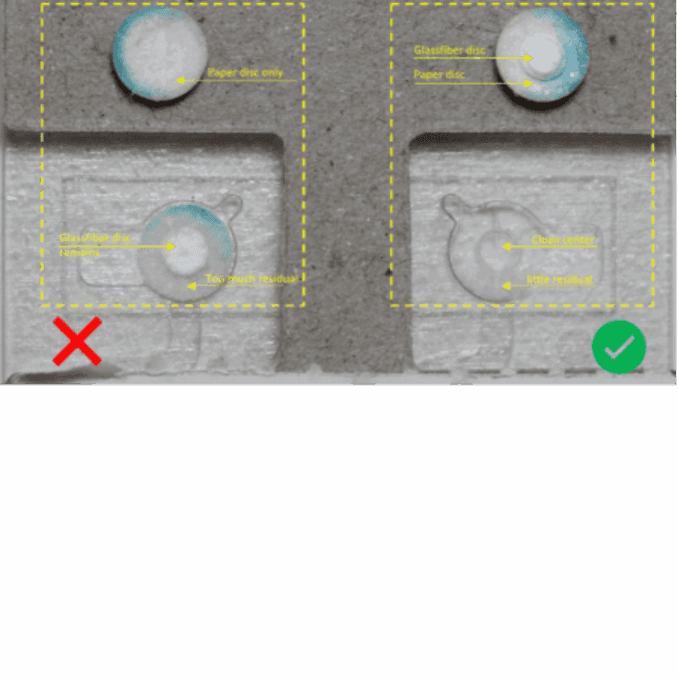
After collection of the disc, the sample is eluted. The choice of elution protocol and buffer is dependent on your analyte and downstream analysis. Published methods with conventional DBS samples are also applicable for Capitainer®qDBS and could serve as a guideline.
Standard Procedure:
Add your buffer of choice to the tubes or plate wells containing the discs. 100-150µl is a guide, but must be adjusted depending on concentration and volume requirements. This gives a 10-15 times sample dilution to calculate for in downstream analysis. The discs absorbs approximately 20µl diluent that will not be recovered with the standard protocol. We do not recommend lower elution buffer volume than 50µl for an efficient elution process.
Gently agitate the tube/plate containing disc and extraction buffer for 30-60 minutes and then transfer the liquid to a clean tube or plate.
With Spin Filter Tubes/Plates:
To reduce the diluent volume and introduce a clean-up step, the use of spin columns/plates to elute the sample might be considered. This has the benefit of also recovering most of the 20µL liquid absorbed by the disc.

1.Choose appropriate filter for your analyte
2.Add the qDBS disc to the column
3.Apply buffer, gently agitate for 60 min
4.Spin down the eluate, discard column + disc
For automation setup, the use of this extraction principle has the benefit of producing an eluate without the DBS disc remaining at the bottom of the wells. This means that pipetting robots potentially can go down deeper in the well and thereby pipette from lower volumes.
Certain liquid handling robots can also handle filter plates, thereby enabling a fully automated workflow for assay setup downstream of the disk removal.