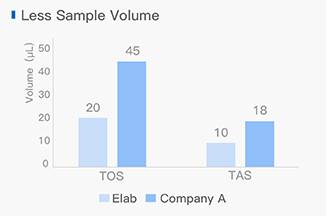
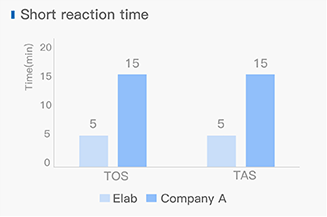
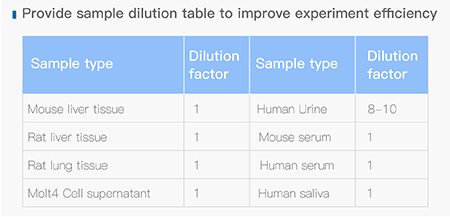
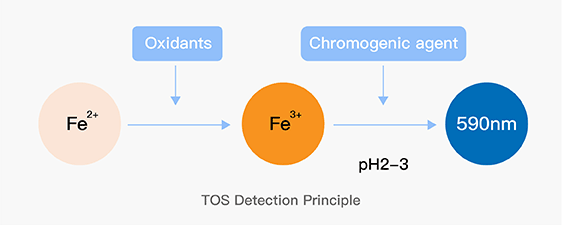
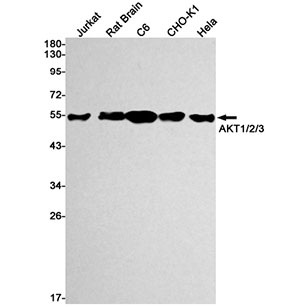
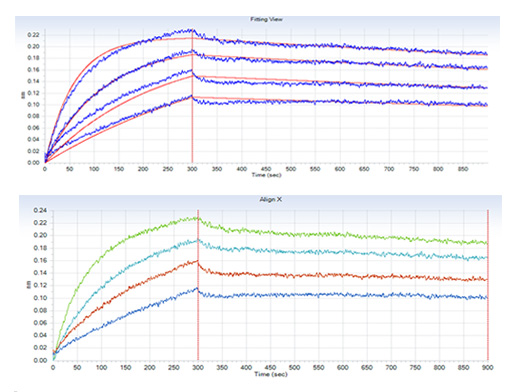
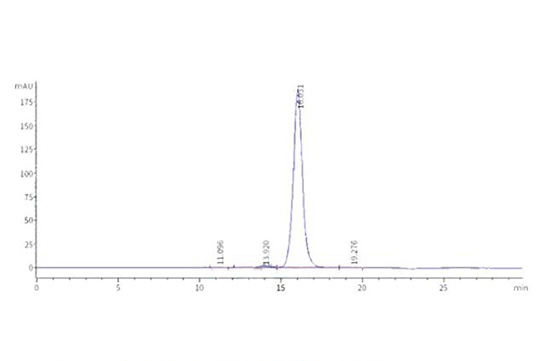
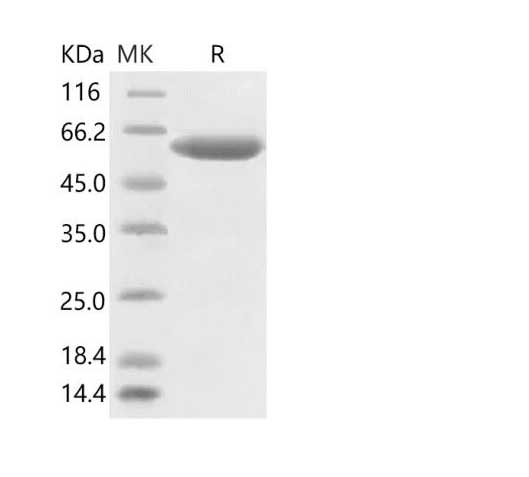
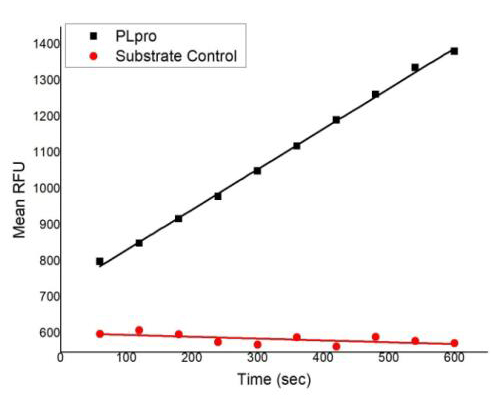
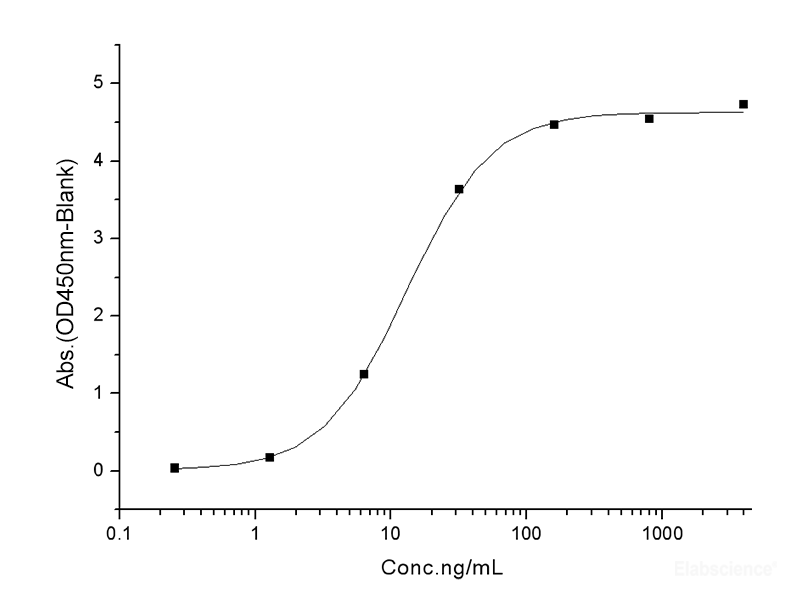
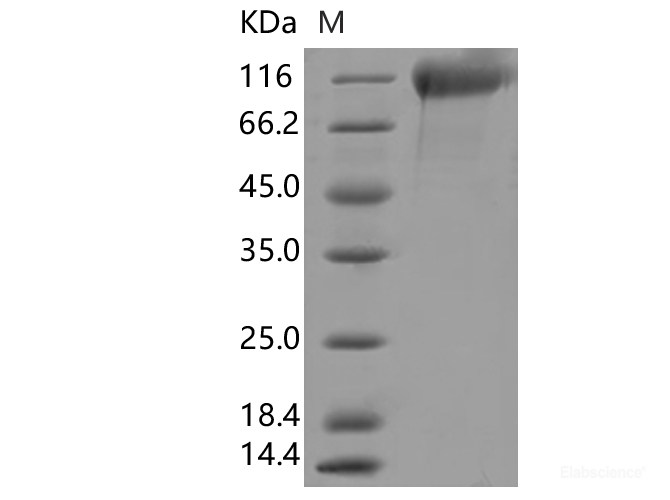
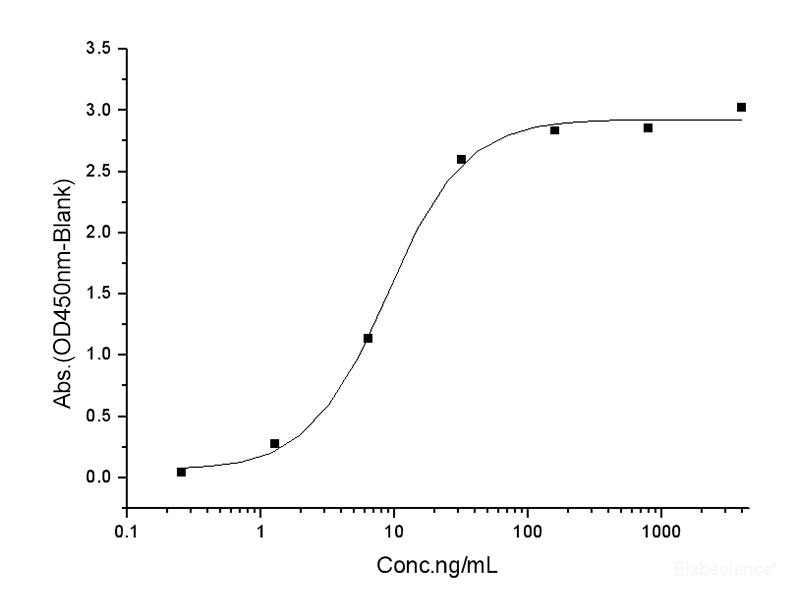
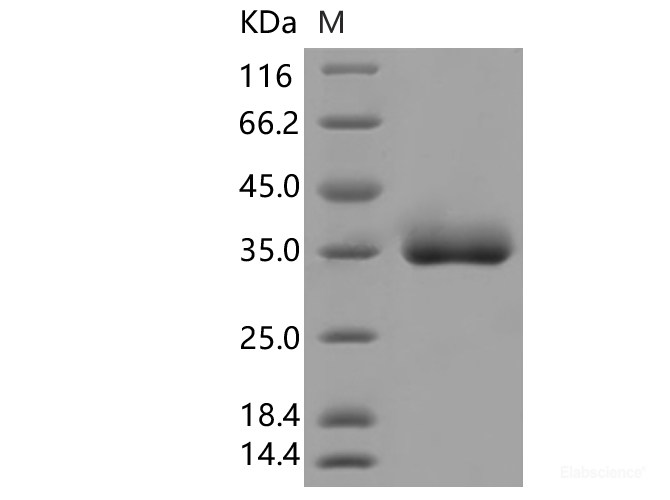
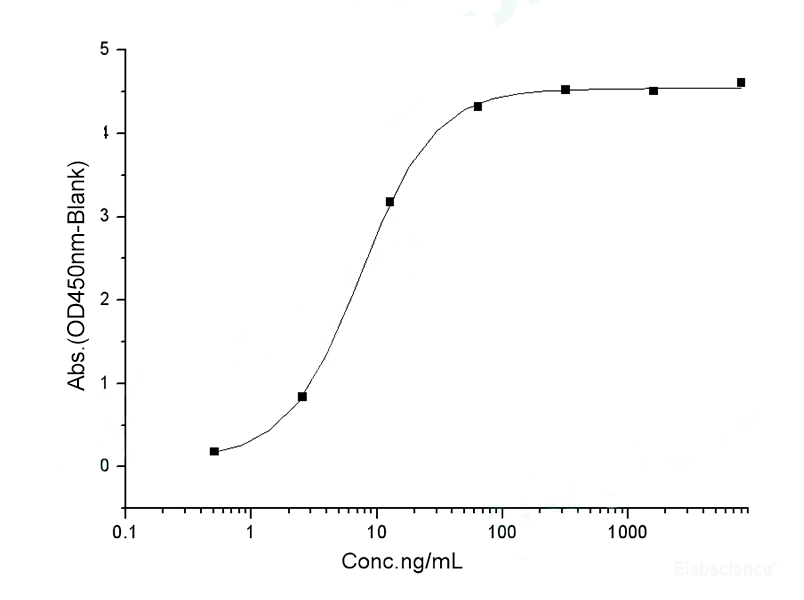
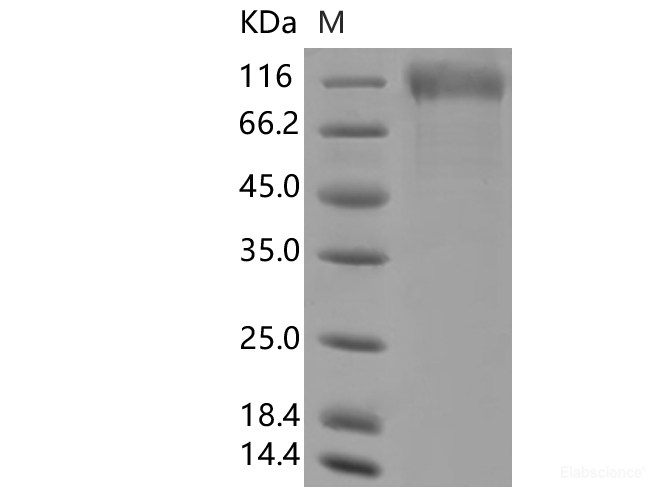
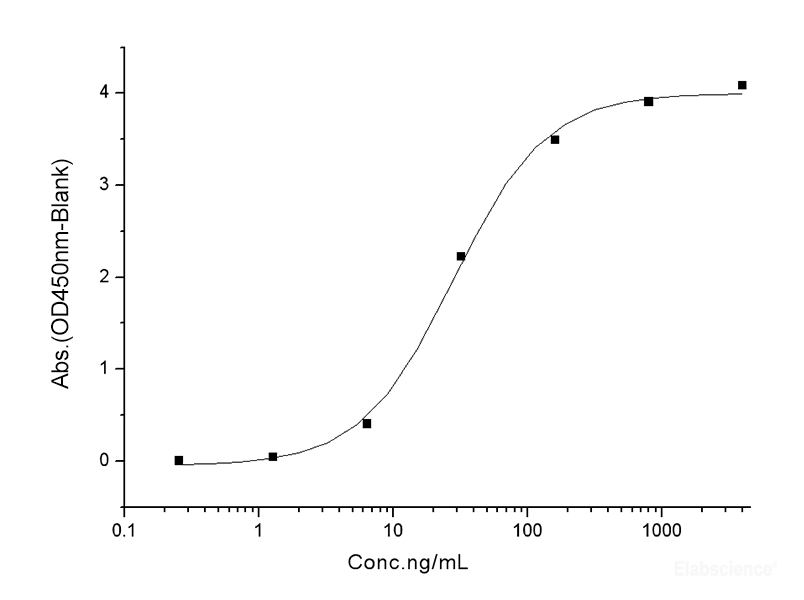
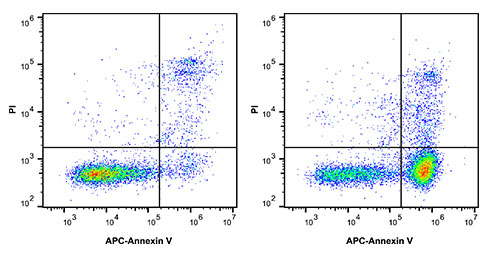
Dissolving a polypeptide is a very complex process, and it is generally difficult to determine a suitable solvent quickly. Usually take a little for a preliminary test, and do not dissolve completely before determining the proper solvent.
The following methods can help you choose the right solvent:
Determine the charge specificity of the polypeptide, set the acidic amino acid Asp (D), Glu (E) and C-terminal COOH to -1; basic amino acid Lys (K), Arg (R), His (H) and N-terminus NH2 is +1 and the charge of the other amino acids is zero. Calculate the net charge number.
If the net charge number is >0, the polypeptide is alkaline and dissolved in water; if the peptide does not dissolve, add 10% acetic acid dropwise with vortexing. The peptide solution can also be warmed slightly. Longer peptides (over 20 amino acids) with a small overall net charge might require the addition of a stronger acid. Trifluoroacetic acid (TFA 10-50 µL) is often used to solubilize peptides but it is not cell-friendly and thus is used only when acetic acid fails to help solubilize the peptide. After the addition of TFA, the peptide should be diluted to approximately 1 mL with deionized water.
If the net charge number is <0, the peptide is acidic and dissolved in water; If the peptide does not dissolve, add ammonium hydroxide (NH4OH 10-50 µL), and dilute the peptide to approximately 1 mL with deionized water.
Note: Caution must be used, however, with peptides that contain cysteine (C), as the used of alkaline pH can cause disulfide bond formation.
If the net charge number = 0, the polypeptide is neutral and generally needs to be dissolved with an organic solvent such as acetonitrile, methanol or isopropanol, DMSO and the like. It has also been suggested that urea is required to dissolve highly hydrophobic polypeptides.