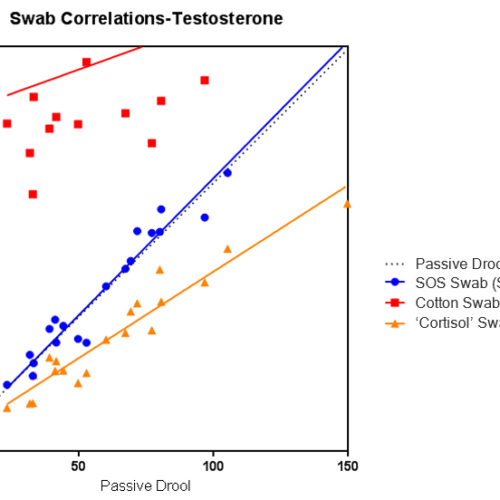
The Salimetrics Salivary IL-1 Beta (IL-1B, Interleukin-1 beta, IL-1β) Enzyme Immunoassay Kit was specifically designed to standardize the detection of IL-1 Beta in saliva samples for research and biomedical laboratories. Using a small sample volume, this assay kit has an extended range that spans the expected IL-1 Beta levels found in human saliva. The average inter- and intra-assay precision coefficients of variation are low with no deleterious matrix effects often found in saliva which are characterized through dilution- and spike-recovery validation procedures. This IL-1 Beta assay kit has also been formatted to minimize cross reactivity for related cytokines.Interleukin-1 Beta is widely accepted as a biomarker of systemic inflammation. IL-1 Beta serves an important role in activating the production of other pro-inflammatory cytokines such as TNF-α, IL-6, and IL-1α, as well as inducing the acute phase response. IL-1 Beta is considered the prototypic ‘multi-functional’ cytokine, affecting nearly all cell types, either alone, or in combination with other cytokines. IL-1β and its associated signaling systems may also play a role in mediating the response of the circadian timing system to immune challenge, as well as the basal functioning of the SCN clock. IL-1 Beta is present in the saliva of both healthy and diseased individuals. The sources of IL-1 Beta production in the oral cavity include macrophages, monocytes, fibroblasts, and mucosal epithelial cells. IL-1 Beta has also been found to be synthesized and released from acinar and ductal cells in mouse salivary glands. Human tear fluid and gingival crevicular fluid (GCF), which may be present as components of whole saliva, also contain IL-1β.
Levels of IL-1 Beta in saliva and CGF have been studied in relation to gingival and periodontal disease, as well as in relation to other oral diseases such as Sjögren’s syndrome and oral cancer. Levels of IL-1 Beta in saliva and GCF have also been observed to change in response to various types of physical and psychological stressors, similar to the response seen in the circulation. Additionally, IL-1 Beta levels in saliva and GCF have been reported to have a circadian rhythm. Dysregulation of IL-1 Beta synthesis or control mechanisms can be involved in numerous disease processes. Studies in humans report that IL-1 Beta levels are generally higher in saliva than in plasma or serum, and serum/plasma levels are often below the limit of detection. Correlation of IL-1 Beta levels in blood and saliva has not been reported in humans, but blood-saliva correlations for other pro-inflammatory cytokines, such as IL-6, have generally been found to be only modest. IL-1 Beta has also been found to be released from mouse parotid acinar cells in response to alpha- and beta-adrenergic stimulation.