Methods of Emulsification
TiterMax®Research Adjuvant combines the benefits of a potent synthetic adjuvant (copolymer CRL-8941) with those of a microparticulate-stabilized, water-in-oil emulsion containing a metabolizable non-toxic oil, squalene. Emulsions of TiterMax®can be prepared using any technique which works with Freund’s Complete Adjuvant (FCA). There are several methods from which you may choose to emulsify your antigen, depending on the equipment you have available and the volume of emulsion you are going to prepare. The two methods we find to be the best in terms of simplicity and recovery of emulsion are the two syringe, double hub emulsifying needle method and the Kontes Pellet Pestle® homogenizer method. The latter is especially suited to small volumes. A number of other methods suggested by recent TiterMax® users are also described. Each of these methods will produce stable water-in-oil emulsions in 1 to 5 minutes.
Stable water-in-oil emulsions are notoriously difficult to make. The TiterMax® formulation was developed to produce a very stable emulsion. TiterMax®was developed to meet the specific needs of investigators for an immunoadjuvant that is at least as effective as Freund’s Complete Adjuvant (FCA), and safer and easier to use. Like FCA, TiterMax® can be used with a wide variety of antigens. Please follow the step-by-step instructions carefully for the method you choose.
Stability of the TiterMax®-in-Oil Emulsion
To test whether your TiterMax® emulsion is ready to use, expel a tiny drop onto the surface of water. It should expel from the syringe with a consistency similar to whipped cream and should hold together on the surface of water. If you are preparing a small volume of emulsion, you may touch the tip of a pipette or applicator stick to your emulsion preparation and submerge it into water. Either way, the emulsion should hold together. In the event that the emulsion disperses on or in the water, reconnect the syringe (or repeat small volume procedure) and emulsify for another minute.
Stability of the Emulsion after Prolonged Storage
A 50:50 water-in-oil emulsion can usually be stored at room temperature, 4° C , -20° C or -70° C for as long as your antigen is stable. Upon storage, approximately 20% of the oil will disassociate from the emulsion. You may leave the emulsion in a syringe and simply re-emulsify when you are ready to use again for injecting. The stability of your antigen must be considered to do this. TiterMax® emulsions have been repeatedly frozen and thawed or left them at room temperature for several weeks.
Reagents that May Interfere with Emulsification or Stability
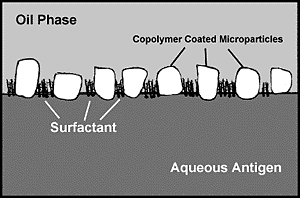
Immunogens which contain high concentrations of surfactants or other materials may interfere with emulsification. We have found that SDS, which may be present in acrylamide gels, in concentrations > 1% or urea in concentrations > 1.0 M significantly reduces the emulsifying capacity of TiterMax®. Other similar materials are likely to have the same effect. Some surface active agents serve as demulsifying agents which break emulsions. The modern emulsifiers and microparticulate stabilizers of TiterMax® are able to overcome the effects of most such agents present in moderate quantities.
Method 1: Two-Syringe, Double Hub Needle
This method is suitable for emulsion volumes between 1 ml and 10 mls. Emulsion recovery is approximately 80 to 90% when preparing volumes of 1 to 10 mls and approximately 50 to 60% when preparing volumes of <0.5 ml.
Materials:
TiterMax®Research Adjuvant
Two 3.0 ml all-plastic syringes
One 18 gauge needle for withdrawing TiterMax® from the vial, or use syringe without needle or positive displacement precision pipette if you open entire vial
One 18 gauge double hub emulsifying needle
Antigen in saline or other suitable fluid (typical dose range in mice is 15 to 125 µg/mouse)
Procedure:
NOTE: Prior to preparation of a TiterMax® water-in-oil emulsion, warm the TiterMax® to room temperature and vortex for 30 seconds. Make sure the TiterMax®is a homogeneous suspension of copolymer-coated microparticles before proceeding to emulsify by any method.
For 1 ml of a 50:50 water-in-oil emulsion you will need 0.5 ml of the aqueous antigen and 0.5 ml of TiterMax® .
- After TiterMax®has been vortexed, load a syringe with 0.5 ml TiterMax® . Load the second syringe with 0.25 ml of antigen in aqueous medium. Set aside the other 0.25 ml of antigen. NOTE: It is important to add the aqueous antigen phase to the TiterMax® in at least 2 small volumes.
- Connect the two syringes via an 18 gauge double hub emulsifying needle. Mix the TiterMax® with the antigen by forcing the materials back and forth through the needle for approximately 1 minute. NOTE: It is important to push the antigen into the TiterMax®syringe first, so that the aqueous phase enters the oil phase rather than vice versa. Hold the syringes carefully so that they do not come apart from the double hub needle during emulsification. The formation of a water-in-oil emulsion is signaled by a sudden increase in viscosity, i.e., more force is required to move the material through the needle. NOTE: After ~ 1 minute a whipped-cream-like water-in-oil emulsion forms. Push all of the emulsion into one syringe and disconnect the empty syringe.
- Load the empty syringe with the remaining 0.25 ml aqueous antigen solution. Reconnect the syringes and emulsify for another 30 to 60 seconds. NOTE: Again, first push the antigen into the water-in-oil emulsion. Care must be taken in holding the syringes together since the oil may lubricate and loosen the connection. That is why it is preferable to use a lock tip syringe. Push all of the emulsion into one syringe and disconnect the empty syringe. If necessary, load the syringe you have chosen for injecting your species of animals.
- To test for stability, place a drop of emulsion on water.
Precautions:
The syringes should be all plastic or siliconized glass. Plastic syringes with rubber pistons contain a lubricant which fails in the presence of TiterMax® and causes the syringes to stick. Use caution not to loosen the syringes from the double hub needle during emulsification. This will cause you to lose the emulsion.
Method 2: Two-Syringe, 3-Way Stopcock
This method is suitable for emulsion volumes between 1 ml and 10 mls. Available 3-way stopcocks have larger bores than the 18 gauge double hub needles (Method 1) so that emulsification takes longer and the syringes connected to 3-way stopcocks are often more difficult to hold. Recovery of emulsion is approximately 70 to 80%.
Materials:
TiterMax®Research Adjuvant
Two 3.0 ml all plastic or siliconized glass syringes (preferably lock tip)
One 18 gauge needle for withdrawing TiterMax® from the vial, or syringe without needle or positive displacement precision pipette if you open entire vial
One 3-way plastic disposable or stainless steel reusable stopcock
Antigen in saline or other suitable fluid (typical dose range in mice is 15 to 125 µg/ mouse)
Procedure:
NOTE: Prior to preparation of a TiterMax®water-in-oil emulsion, warm the TiterMax® to room temperature and vortex for 30 seconds. Make sure the TiterMax® is a homogeneous suspension of copolymer-coated microparticles before proceeding to emulsify by any method.
For 1 ml of a 50:50 water-in-oil emulsion you will need 0.5 ml of the aqueous antigen and 0.5 ml of TiterMax®.
- After TiterMax®has been vortexed, load a syringe with 0.5 ml TiterMax®. Load the second syringe with 0.25 ml of antigen in aqueous medium. Set aside the other 0.25 ml of antigen. NOTE: It is important to add the aqueous antigen phase to the TiterMax® in at least 2 small volumes.
- Connect the two syringes via a 3-way stopcock. Mix the TiterMax® with the antigen by forcing the materials back and forth through the stopcock for approximately 1 minute. NOTE: It is important to push the antigen into the TiterMax®syringe first, so that the aqueous phase enters the oil phase rather than vice versa. Hold the syringes carefully so that they do not come apart from the 3-way stopcock during emulsification. NOTE: After approximately 1 minute a whipped-cream-like water-in-oil emulsion forms. Push all of the emulsion into one syringe and disconnect the empty syringe.
- Load the empty syringe with the remaining 0.25 ml aqueous antigen solution. Reconnect the syringes and emulsify for another 30 to 60 seconds. NOTE: Again, first push the antigen into the water-in-oil emulsion. Care must be taken in holding the syringes together since the oil may lubricate and loosen the connection. It is preferable to use a lock tip syringe. Push all of the emulsion into one syringe. Disconnect the empty syringe and connect the syringe you have chosen for injecting for filling. Alternatively, simply disconnect the full syringe and add the appropriate needle for injecting animals.
- To test stability, place a drop of emulsion on water.
Precautions:
The syringes should be siliconized glass or all plastic. Plastic syringes with rubber pistons contain a lubricant which fails in the presence of TiterMax® and causes the syringes to stick. Use extreme caution during emulsification so that you do not loosen the syringes from the 3-way stopcock. This will cause you to lose the emulsion.
Method 3: One Syringe, Blunt Needle
This method is useful for volumes less than 0.5 ml and has been used with volumes as low as 0.05 ml final emulsion volume. Recovery of emulsion is approximately 50 to 75%.
Materials:
TiterMax®Research Adjuvant
1 ml all-plastic syringe
18 gauge x 1.5 inch blunt needle for emulsifying
1.5 ml conical bottom plastic centrifuge tube
One 18 gauge needle for withdrawing TiterMax® from the vial, or syringe without needle or positive displacement precision pipette if you open entire vial
Antigen in saline or other suitable fluid (typical dose range in mice is 15 to 125 µg/ mouse)
Procedure:
NOTE: Prior to preparation of a TiterMax® water-in-oil emulsion, warm the TiterMax® to room temperature and vortex for 30 seconds. Make sure the TiterMax®is a homogeneous suspension of copolymer-coated microparticles before proceeding to emulsify by any method.
For 200 µl of a 50:50 water-in-oil emulsion you will need 100 µl of the aqueous antigen and 100 µl of TiterMax®.
- Grasp the pointed end of the needle with pliers and gently bend it back and forth until the tip breaks off producing a 1 inch blunt end needle. Attach the blunt 18 gauge needle to a 1 ml all-plastic syringe.
Add 100 µl of TiterMax®adjuvant to the 1.5 ml centrifuge tube.
- Add 50µl of your antigen solution. The antigen-adjuvant mixture is drawn into the syringe and expressed back into the tube several times until a thick white emulsion forms. Add the remaining 50µl of your antigen solution and repeat the process. NOTE: Certain technical points are important. The air drawn into the syringe during the process does not impede the emulsification process. In approximately 1 minute, the entire material will be transformed into a water-in-oil emulsion. If one is careful not to smear the material on the sides of the tube, it can be drawn almost quantitatively into the syringe (using the 18 gauge needle). If you get emulsion on the sides of the tube, centrifuge at low speed (100 x g) for 2 minutes to pellet the emulsion. Remove the blunt needle and replace with a suitable needle for injecting.
- To test stability, place a tiny drop of emulsion on or in water.
Method 4: Emulsifying in Syringe Using Sonication
This method is suitable for emulsion volumes between 0.5 ml and 2 ml. Recovery of emulsion is approximately 80 to 90%.
Materials:
TiterMax®Research Adjuvant
One 18 gauge needle for withdrawing TiterMax® from the vial, or syringe without needle or positive displacement precision pipette if you open entire vial
One syringe for preparation of emulsion and immunization; piston removed; tip sealed with Parafilm®
Parafilm® for sealing tip of syringe
Antigen in saline or other suitable fluid (typical dose range in mice is 15 to 125 µg/ mouse)
Sonic dismembrator with microtip
Procedure:
NOTE: Prior to preparation of a TiterMax® water-in-oil emulsion, warm the TiterMax® to room temperature and vortex for 30 seconds. Make sure the TiterMax®is a homogeneous suspension of copolymer-coated microparticles before proceeding to emulsify by any method.
For 1.0 ml of a 50:50 water-in-oil emulsion you will need 0.5 ml of the aqueous antigen and 0.5 ml of TiterMax®.
- Carefully seal the tip of the syringe with Parafilm®. After TiterMax®has been vortexed, load a 2.0 ml syringe with 0.5 ml TiterMax®. Add 0.5 ml of antigen in aqueous medium. NOTE: It is not necessary to add the aqueous antigen in small volumes when using this method.
- Place microtip of sonicator into syringe and turn on power. After ~ 35 to 45 seconds a whipped-cream-like water-in-oil emulsion forms. NOTE: Care must be taken to seal the syringe. Push all of the emulsion together by tapping as you insert the piston.
- To test stability, place a small amount of emulsion on or in water.
Precautions:
Approximately 10 to 20% of the emulsion sticks to the microtip of the sonicator.
NOTE: Sonicators
Other types of sonicators have also been used to prepare water-in-oil emulsions, e.g. the Bransonic 32. Using the Bransonic takes several minutes longer and requires extra care to ensure the syringe is sealed and protected from the water.