Reactive oxygen species (ROS), such as superoxide anion (O2•–), hydroxyl radical (OH•) and the non-radical species hydrogen peroxide (H2O2), are generated endogenously through the process of mitochondrial oxidative phosphorylation, or they may arise from interactions with exogenous sources such as xenobiotics, cytokines, or bacterial invasion.1
At moderate levels, ROS is essential for normal cellular signaling. However, in excess, ROS can oxidize cellular macromolecules such as DNA, lipids, and proteins and ultimately lead to four different cellular fates- apoptosis, necrosis, autophagy, or senescence. These processes negatively affect normal cellular homeostasis, eventually leading to the development of age-associated pathologies and can adversely impact an organism’s life span. ROS stress has been considered a contributing factor in aging and various neurological disorders, including Alzheimer’s disease (AD) and amyotrophic lateral sclerosis (ALS).
A large amount of oxygen being consumed in the brain leads to excessive production of ROS. Most neuron cells have a large membrane enriched in polyunsaturated fatty acids, highly susceptible to ROS.
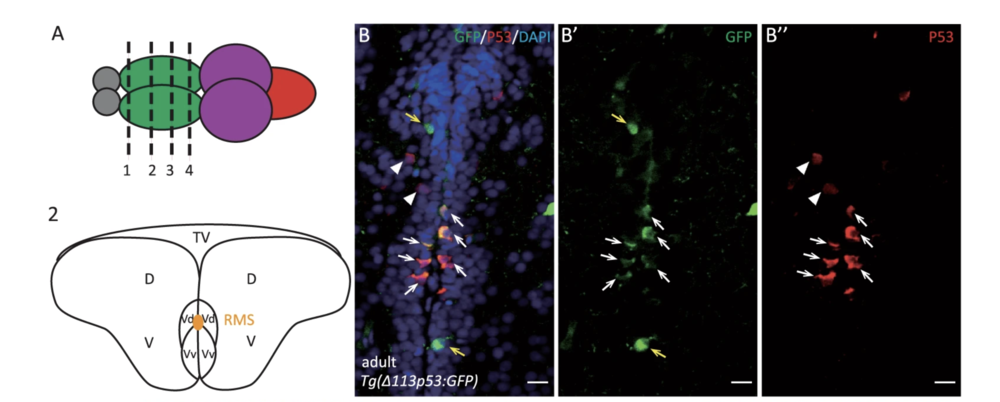
The signaling pathway of the tumor repressor p53 plays a crucial role in response to oxidative stress. In response to high oxidative stress levels, p53 triggers apoptotic activity by upregulating the expression of pro-oxidative genes and apoptotic genes. In response to low-level ROS stress, the expression of Δ133p53, a human p53 isoform, is upregulated to promote cell survival and protect cells from senescence by enhancing the expression of antioxidant genes. In normal conditions, the basal expression of Δ133p53 prevents human fibroblasts, T lymphocytes, and astrocytes from replicative senescence. Brain tissues from AD and ALS patients showed decreased Δ133p53 expression.
Zhao, T., et al. used the zebrafish model to investigate the function of Δ113p53 in brain aging. They found zebrafish Δ113p53 is mainly expressed in some of the radial glial cells along the telencephalon ventricular zone in a full-length p53-dependent manner.