Diffuse midline gliomas: background and emerging therapies
Diffuse midline gliomas (DMG)—high-grade tumors of the primary nervous system that affect children almost exclusively—are considered to be incurable (Huang et al. 2017). Despite decades of clinical trials, the median overall survival rate is just 9–11 months. Tumors are generally considered to be inoperable owing to location and the diffuse and highly infiltrative nature of these neoplasms (Findlay and Redfield 2009; Huang et al. 2017). Until recently, radiation therapy has been the only treatment option to slow the progression of these tumors but has not improved overall survival rates.
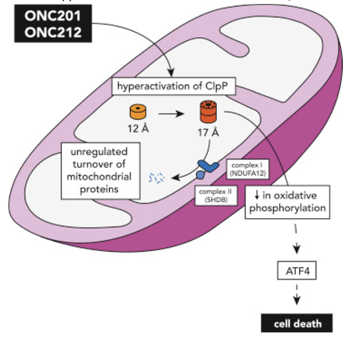
Recent studies that have characterized the molecular features of high-grade pediatric glioma subgroups have identified numerous potential therapeutic targets (Chi et al. 2019; Sturm, Pfister, and Jones 2017). As DMGs frequently exhibit altered energy metabolism, mitochondrial reprogramming, and reduced copy number of mitochondrial DNA in tumor cells, mitochondrial pathways have emerged as pharmaceutical targets for pediatric high-grade gliomas (Shen et al. 2020; Przystal et al. 2022; Bonner et al. 2021). Recently, a new class of anti-cancer small molecules, called imipridones, that target G protein-coupled receptors (GRPCs)—the largest class of cell surface receptors in humans—have demonstrated clinical efficacy in patients with advanced solid tumors and hematological cancers (Prabhu et al. 2020). Specifically, the imprinidones ONC201, 206, and 212 have been shown to exhibit clinical efficacy in a broad range of tumors by hyperactivating the proteolytic activity of the proteolytic subunit of the highly conserved serine protease mitochondrial caseinolytic peptidase P (ClpP) (Prabhu et al. 2020; Wagner et al. 2017; Bonner et al. 2021), which plays a key role in protein quality control in the mitochondrion by degrading misfolded proteins and has been shown to be the most significant predictive biomarker of response to treatment with imprinidones (Bonner et al. 2021; Przystal et al. 2022) (Figure 1).
Figure 1. Graphical illustration of the hyperactivation of ClpP by imprinidones ONC201 and ONC212 in the mitochondria, which leads to the unregulated turnover of mitochondrial proteins, decreased oxidative phosphorylation, and ultimately cell death. Figure adapted from Wang and Dougan (2019).
Elucidating the anti-cancer mechanisms of ONC201 and ONC206 in DMGs
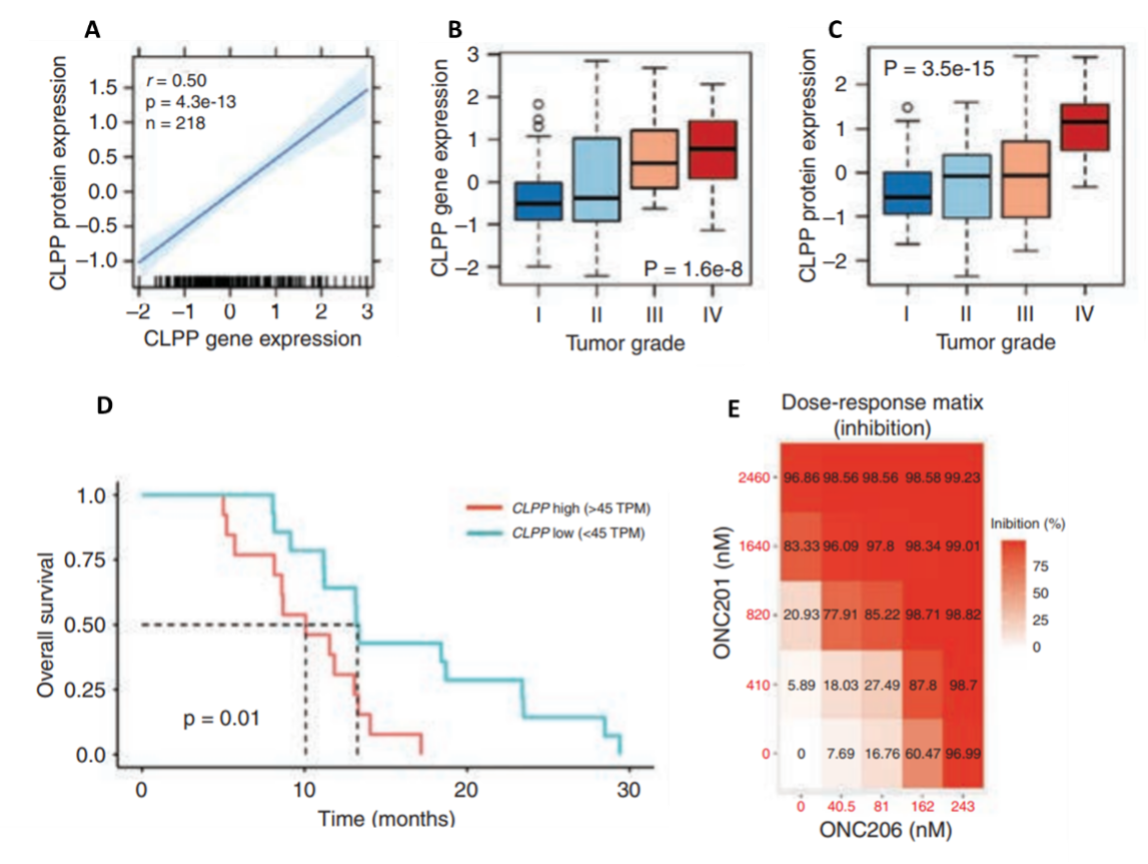
While studies that demonstrate the clinical efficacy of imprinidones in a wide range of cancers are underway, the underlying molecular mechanisms of ONC201 and its derivative ONC206 in DMGs are still unclear. A recent study by Przystal et al. (2022) sought to elucidate the effects of ONC201 and ONC206 on tumor bioenergetics in DMG models. An analysis of ClpP expression and protein levels from the Pediatric Brain Tumor Atlas (PBTA) and Clinical Proteomic Tumor Analysis Consortium (CPTAC) revealed a positive correlation between ClpP protein and mRNA expression, as well as a significant association between both ClpP mRNA and protein expression and glioma grade, where elevated ClpP expression and protein levels were significantly correlated with lower patient survival (Figure 2 A–D). The authors also found that all human primary DMG cell lines responded to treatment with ONC201 and ONC212 in both nano- and micromolar concentrations (Figure 2E). Moreover, using CRISPR/Cas9 to knock out ClpP in DMG cells confirmed the crucial role of ClpP in treating DMG cells with imipridone.
Figure 2. Correlations between ClpP regulation and characteristics of high-pediatric central nervous system tumors and the effects of ONC201 and ONC206 on human primary DMG cell lines. A) Correlation between ClpP protein levels and mRNA expression. B) Association between ClpP protein expression and tumor grade. C) Association between ClpP mRNA expression and tumor grade. D) Relationship between ClpP mRNA expression and overall survival. E) Inhibition as a function of treatment dose of both ONC201 and ONC 206 in human primary DMG cell lines. These results highlight the additive and synergistic effects of combining both therapies. TPM: transcripts per kilobase million.
Przystal et al. (2022) observed that protein levels of both integrated stress response biomarkers (phospho-eIF2α, ATF4, DR5, and CHOP) and apoptosis markers (cleaved caspase-7 and -3, PARP, and XIAP) were increased in response to treatment with both drugs. Furthermore, the authors confirmed that ONC201 and ONC206 suppress DMG cell growth via metabolic reprogramming, as is evidenced by decreases in both mitochondrial respiration and ATP production, as well as changes in glycolysis.
RNA profiling paints a broad picture of molecular pathways involved in imipridone treatment
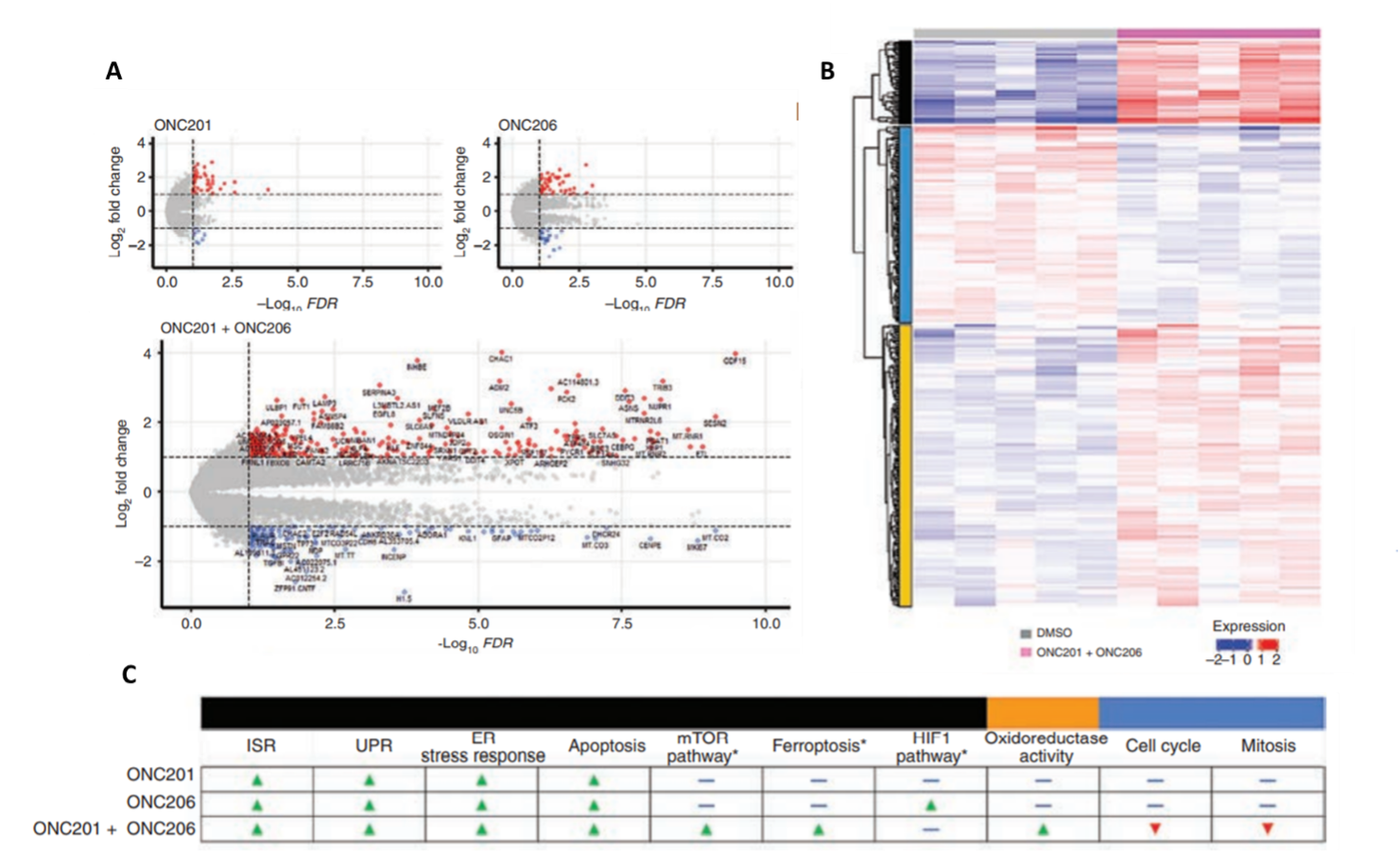
To elucidate the molecular targets of ONC201 and ONC206, to corroborate the aforementioned changes in protein levels and cellular metabolism, and to further examine the combined effects of both drugs, Przystal et al. (2022) used BRB-seq to observe transcriptomic changes in response to drug treatment in DMG human cell lines. The BRB-Seq library was prepared using the Mercurius BRB-seq kit for 96 samples and sequenced to a depth of 441.4 million reads per sample using NextGen sequencing. The findings of differential gene expression analyses confirmed that the integrated stress response pathway was indeed upregulated (i.e., ATF4, CHOP, etc), in which the strongest effect was a result of combined ONC201/6 treatment. BRB-seq also enabled the authors to observe distinct transcriptional reprogramming in response to both individual drug therapies and combined treatment in DMG cells (Figure 3).
Figure 3. BRB-seq reveals transcriptional reprogramming in diffuse midline glioma cells treated with ONC201 and ONC206. A) Differential gene expression as a result of treatment with either ONC201, ONC206, or ONC201 + ONC206 as demonstrated by bulk RNA sequencing (red: upregulation, blue: downregulation). B) ONC201/6 combined treatment resulted in significant levels of differential gene expression (relative to the DMSO control). Functional enrichment analyses (i.e., Gene Ontology, KEGG) identified molecular pathways uniquely upregulated (red) and downregulated (blue) in response to each treatment condition. The black, yellow, and blue bars on the left correspond to categories of molecular pathways show in panel C. C) Differentially regulated molecular pathways in response to individual and combined treatments.
Bulk RNA profiling also showed that the combined ONC201/6 treatment resulted in the significant upregulation of more than 200 unique transcripts, including genes involved in homeostatic pathways for metabolic function and oxidation-reduction (e.g., mTOR pathway, oxidoreductase activity, ferroptosis pathways), as well as apoptosis. Interestingly, treatment with ONC206 resulted in a significant upregulation of the hypoxia-inducible factor-1 pathway, which could explain the increase in glycolysis observed in response to ONC206 treatment. Perhaps most notably, genes involved in cell cycle and mitosis were observed to be downregulated in response to the combined treatment (Figure 3C). In conclusion, transcriptome-wide analyses were in agreement with drug responses observed at the biochemical and cellular levels, supporting the notion that ONC201 and ONC206 work not only additively but synergistically, and highlighted important molecular pathways as targets for further investigation.
The findings of Przystal et al. (2022) confirm the key role of ClpP in the efficacy of imipridone in treating DMG and elucidate the molecular pathways both up- and downregulated in response to treatment. Most importantly, this study paves the way for Phase I clinical trials for the use of ONC201 and its derivatives in children, to ultimately gain traction in treating—and hopefully curing—this devastating pediatric cancer.
To find out more about how BRB-seq could help your study, please contact us at info@stratech.co.uk
References
- Bonner, Erin R., Sebastian M. Waszak, Michael A. Grotzer, Sabine Mueller, and Javad Nazarian. 2021. “Mechanisms of Imipridones in Targeting Mitochondrial Metabolism in Cancer Cells.” Neuro-Oncology 23 (4): 542–56. https://doi.org/10.1093/neuonc/noaa283.
- Chi, Andrew S., Rohinton S. Tarapore, Matthew D. Hall, Nicole Shonka, Sharon Gardner, Yoshie Umemura, Ashley Sumrall, et al. 2019. “Pediatric and Adult H3 K27M-Mutant Diffuse Midline Glioma Treated with the Selective DRD2 Antagonist ONC201.” Journal of Neuro-Oncology 145 (1): 97–105. https://doi.org/10.1007/s11060-019-03271-3.
- Findlay, W. A., and R. J. Redfield. 2009. “Coevolution of DNA Uptake Sequences and Bacterial Proteomes.” Genome Biology and Evolution 1 (January): 45–55. https://doi.org/10.1093/gbe/evp005.
- Huang, Tina Y., Andrea Piunti, Rishi R. Lulla, Jin Qi, Craig M. Horbinski, Tadanori Tomita, C. David James, Ali Shilatifard, and Amanda M. Saratsis. 2017. “Detection of Histone H3 Mutations in Cerebrospinal Fluid-Derived Tumor DNA from Children with Diffuse Midline Glioma.” Acta Neuropathologica Communications 5 (1): 28. https://doi.org/10.1186/s40478-017-0436-6.
- Prabhu, Varun Vijay, Sara Morrow, Abed Rahman Kawakibi, Lanlan Zhou, Marie Ralff, Jocelyn Ray, Aakash Jhaveri, et al. 2020. “ONC201 and Imipridones: Anti-Cancer Compounds with Clinical Efficacy.” Neoplasia (New York, N.Y.) 22 (12): 725–44. https://doi.org/10.1016/j.neo.2020.09.005.
- Przystal, Justyna M, Chiara Cianciolo Cosentino, Sridevi Yadavilli, Jie Zhang, Sandra Laternser, Erin R Bonner, Rachna Prasad, et al. 2022. “Imipridones Affect Tumor Bioenergetics and Promote Cell Lineage Differentiation in Diffuse Midline Gliomas.” Neuro-Oncology, February, noac041. https://doi.org/10.1093/neuonc/noac041.
- Shen, Han, Man Yu, Maria Tsoli, Cecilia Chang, Swapna Joshi, Jie Liu, Scott Ryall, et al. 2020. “Targeting Reduced Mitochondrial DNA Quantity as a Therapeutic Approach in Pediatric High-Grade Gliomas.” Neuro-Oncology 22 (1): 139–51. https://doi.org/10.1093/neuonc/noz140.
Sturm, Dominik, Stefan M. Pfister, and David TW Jones. 2017. “Pediatric Gliomas: Current Concepts on Diagnosis, Biology, and Clinical Management.” Journal of Clinical Oncology 35 (21): 2370–77.
- Wagner, Jessica, Christina Leah Kline, Marie D. Ralff, Avital Lev, Amriti Lulla, Lanlan Zhou, Gary L. Olson, et al. 2017. “Preclinical Evaluation of the Imipridone Family, Analogs of Clinical Stage Anti-Cancer Small Molecule ONC201, Reveals Potent Anti-Cancer Effects of ONC212.” Cell Cycle 16 (19): 1790–99. https://doi.org/10.1080/15384101.2017.1325046.
- Wang, Shaomeng, and David A. Dougan. 2019. “The Direct Molecular Target for Imipridone ONC201 Is Finally Established.” Cancer Cell 35 (5): 707–8. https://doi.org/10.1016/j.ccell.2019.04.010.