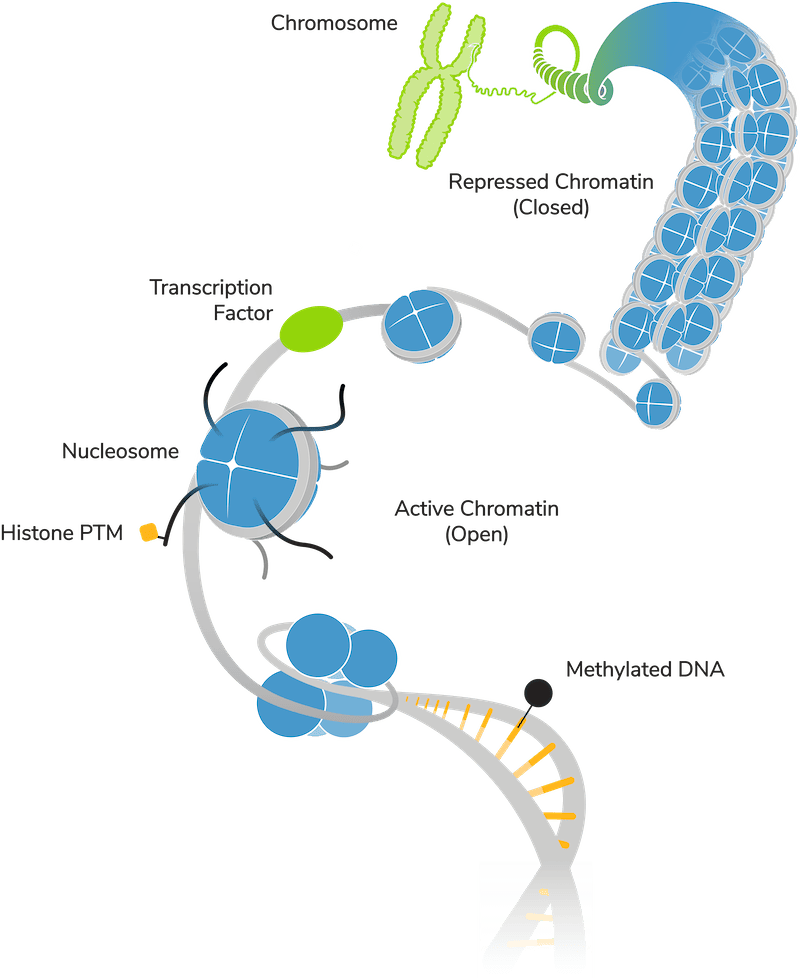
Disease associations
- HDACs are overexpressed in many types of cancers12. In fact, the broad-spectrum HDAC inhibitors vorinostat and romidepsin were among the earliest epigenetic-targeted drugs approved by the FDA for cancer therapy12,13.
- Histone acetylation is also linked to memory formation, aging, and neurodegenerative diseases14,15. HDAC inhibitors have shown promising results in mouse models of Alzheimer’s disease, Huntington’s disease, and others, pointing to new avenues of drug research (15,16.
Histone Methylation: Context-specific regulation of gene expression
Histone methylation, catalyzed by histone methyltransferases, involves the addition of one to three methyl groups to the lysine or arginine residues of histone proteins. Because histone lysine methylation is more widely studied, our review is focused on these marks. For a recent review of arginine methylation, see 17-19.
Unlike acetylation, methylation doesn’t alter the charge of histones. Instead, it provides binding sites for proteins involved in gene regulation, and frequently engages with other histone PTMs and/or DNA methylation through so-called “crosstalk” mechanisms.
Histone methylation has been implicated in various biological processes including DNA repair, X-chromosome inactivation, and cellular differentiation5,20-22. Although histone methylation was thought to be stable for many years, it is in fact reversible – the discovery of the first histone lysine demethylase LSD1 (KDM1A) in 2004 was a major landmark in the field23. Many other lysine demethylases have been discovered in the following years, and LSD1 now represents a major target in cancer drug research24,25.
The effect of histone methylation on gene expression is context-dependent, meaning that it can either facilitate or repress gene expression depending on the site and degree of methylation.
- H3K4me3 is enriched at active transcription start sites (TSSs)5. In mammalian cells, H3K4me3 interacts with TAF3, a subunit of the TFIID transcription factor26. TFIID supports transcriptional initiation via binding to core promoter sequences, such as the TATA box, and acts as a landing site for the RNA Pol II preinitiation complex27. H3K4me3 has also been shown to recruit the SAGA histone acetylation complex, which may promote gene expression28. Despite its localization at TSSs and interactions with core transcriptional machinery, the requirement of
- H3K4me3 for gene expression is an ongoing topic of debate29-31. We recommend reference 31 for additional background.
H3K9me3 denotes constitutively silenced heterochromatin, and its main function is to prevent expression of transposons and other repetitive elements. H3K9me3 also contributes to the development of lamin-associated domains, the term for dense heterochromatin regions tethered to the nuclear lamina32. Notably, H3K9me3 co-occurs with DNA methylation, and the complexes that generate these two features interact with one another to help maintain a condensed chromatin structure5.
- H3K27me3 is enriched at facultative heterochromatin, which means that it associates with repressed genes and enhancers. It is produced by the Polycomb Repressive Complex 2 (PRC2) and interacts with PRC2 to facilitate “spreading” of H3K27me3 into repressed gene bodies. Compared to H3K9me3, this mark occurs in a more permissive chromatin environment, enabling interactions with transcription factors, chromatin readers, and other proteins5.
H3K27me3 is tightly linked to developmental gene expression programs. This mark interacts with Polycomb Repressive Complex 1 (PRC1) to help silence non-lineage specific genes during differentiation22. H3K27me3 is also read by the bromo-adjacent homology (BAH) domain of BAHCC1, which in turn recruits histone deacetylases and other corepressors to maintain silencing33.
Interestingly, H3K27me3 at promoters largely overlaps with unmethylated CpG islands. Loss of DNA methylation leads to redistribution of H3K27me3 across the genome, and re-expression of PRC2 target genes, suggesting DNA methylation helps regulate PRC2 recruitment21,34,35.
Disease associations
Aberrant histone lysine methylation has been associated with various pathologies, highlighting the importance of this epigenetic marker in maintaining cellular homeostasis. A few key examples:
- The histone lysine demethylase LSD1 is overexpressed in many different types of cancers, from solid tumors (e.g. breast, colorectal, prostate) to blood cancers (e.g. acute myeloid leukemia, or AML), underscoring roles in cell differentiation, proliferation, and migration. Several LSD1 inhibitors are in clinical trials, as reviewed in references 24,25.
- Rearrangement and/or gene fusions of the H3K4 methyltransferase MLL1 are a hallmark of acute myelogenous leukemia (AML) and acute lymphoblastic leukemia (ALL)36. More recent work has shown that these fusion proteins also underlie some solid tumors, including lung, intestine, and endometrial cancers37. Researchers are developing inhibitors against some of the most common fusion proteins, with promising results in mice; see reference 37 for further information.
- Mutations in histone methyltransferases also underlie various neurodevelopmental disorders, including Kabuki syndrome, Sotos syndrome, and Wiedemann-Steiner syndrome38. Sotos syndrome is a childhood overgrowth disorder, driven by mutations in the H3K36 methyltransferase NSD1. Sotos syndrome phenocopies Tatton-Brown-Rahman syndrome (TBRS), which is caused by mutations in the DNA methyltransferase DNMT3A.
A 2019 study co-authored by EpiCypher established a mechanistic link between DNMT3A and NSD1, clarifying the similarities between these two diseases and demonstrating another route by which DNA methylation “talks” with histone modifications39. This discovery was supported by our dCypher™ platform, which uses modified nucleosomes to screen chromatin binding specificity in a physiologically relevant context. To learn more about this study and EpiCypher’s dCypher platform, see this blog.