3. Cell morphology and integrity
CUT&RUN requires intact cells with normal morphology. Morphology refers to cell shape and relates to the tissue source and/or how cells grow in culture. Suspension cultures, such as K562 cells, which commonly derive from blood cells and/or cancers, have round and symmetric morphology. Adherent cells can be derived from epithelial, endothelial, neuronal, or fibroblast tissues, and thus display diverse morphology and expansion characteristics. For instance, epithelial cells tend to have a uniform cell shape and grow in patches on cell culture plates, while fibroblasts show asymmetric, elongated morphology, and can be used to study cell migration.
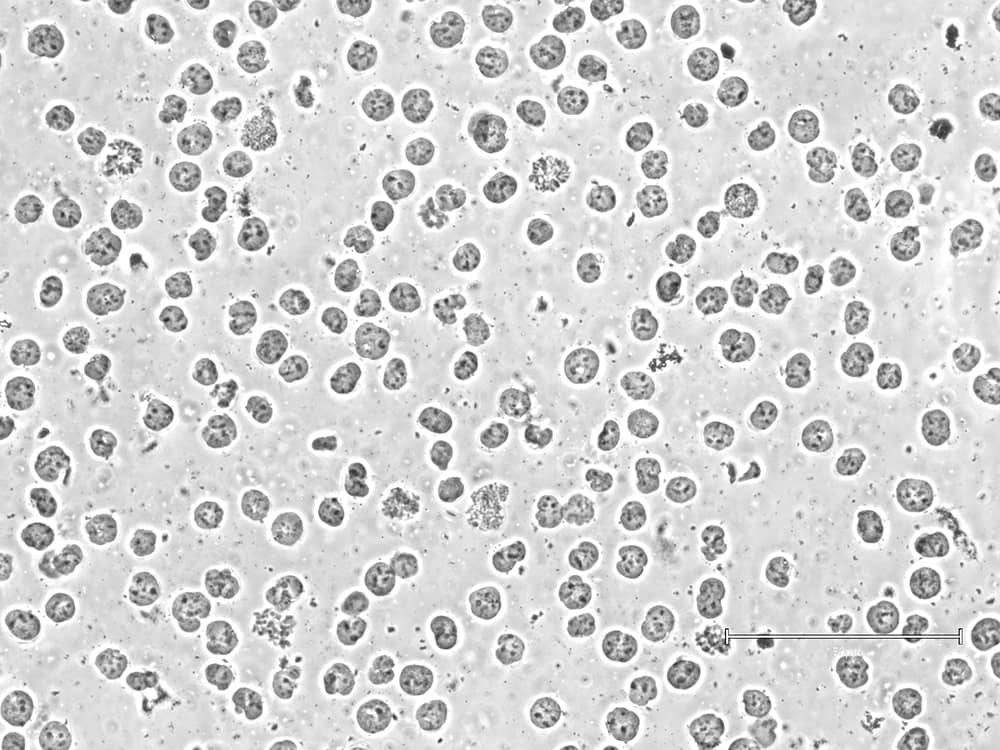
Using lysed or poor-quality cells will lead to equally poor data. To ensure robust sample prep, EpiCypher checks morphology and cell membrane integrity at both initial cell collection and prior to bead binding. The second check is important, as some sample types (e.g. FACS-isolated cells or those from tissues) can be more sensitive to lysis in CUT&RUN Wash Buffers. In these cases, we recommend isolating nuclei for CUT&RUN instead (Figure 1).
Figure 1: Successful isolated K562 cell nuclei. Image by Liz Albertorio-Sáez M.Sc.
4. Cell source considerations: cell lines, primary cells, tissues, and stem cells
Morphology, viability, and cell numbers are directly influenced by the source of your cells. CUT&RUN can be performed using cells from tissues, cell lines, cultured primary cells, or cells purified by other means (e.g. FACS). Here we discuss the advantages and challenges associated with these samples.
Immortalized cell lines
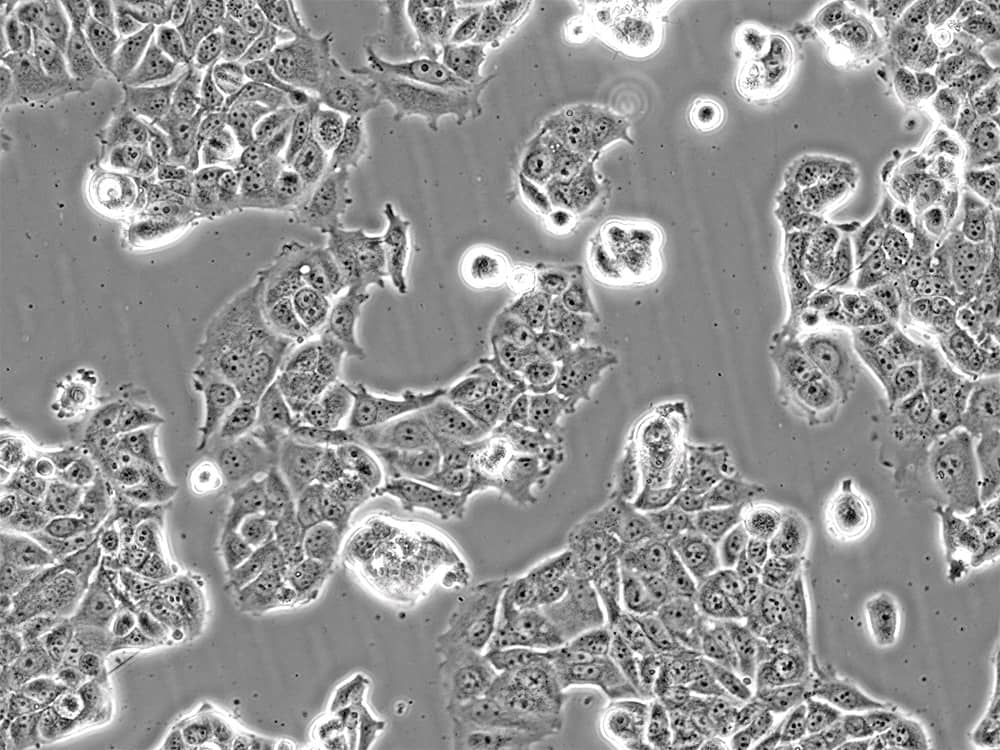
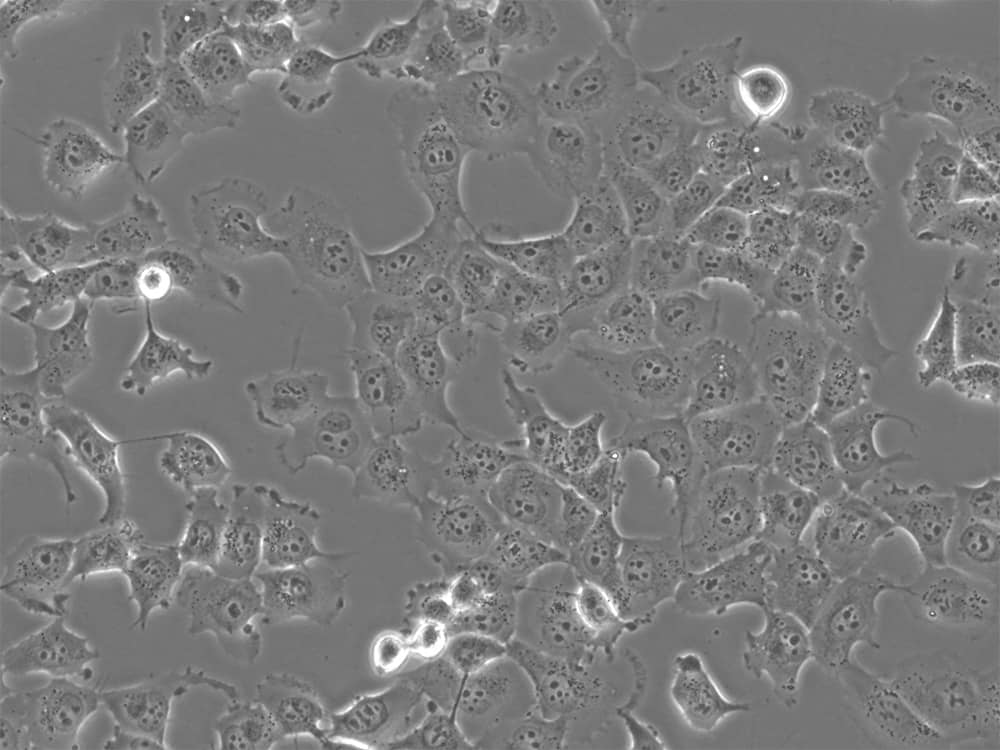
Examples: HEK293, HeLa, K562, NIH/3T3, MCF-7 (Figure 2), H1299 (Figure 3), A549, and THP-1 cell lines.
Advantages: In general, cell lines are the easiest inputs to optimize for CUT&RUN. Cell lines provide:
- Large quantities of cells with high viability – key for CUT&RUN success.
- Robust and highly reproducible CUT&RUN profiling, due to homogeneity of cell lines.
- Robust systems for drug screening, gene overexpression/repression, and more – without destroying sample quality.
Disadvantages: The use of cell lines comes with many caveats, outlined below. However, depending on your experimental goals, cell lines may be the best option.
- In general, cell lines are not representative of in vivo conditions. They are often derived from cancers or other diseased cell populations, and their function may be different compared to primary cells.
- Cell lines are prone to genomic alterations, including chromosomal abnormalities, duplications, and/or genetic drift.
- Contamination with other cell lines is common. Misidentification can hamper assay reproducibility and lead to incorrect biological conclusions.
Figure 2: MCF-7 breast cancer cells in culture. Image by Liz Albertorio-Sáez M.Sc.
Primary cells
Examples: Samples derived from living tissues, either solid (e.g. intestine, lung, liver, skin) or fluid/liquid (e.g. blood), with or without cell culture.
Advantages: Primary cells are frequently used in CUT&RUN, as they enable scientists to:
- Examine chromatin function and biological mechanisms in a native, heterogeneous cellular environment.
- Study the function of rare cell states isolated by FACS or other techniques.
- Determine cellular responses to stimuli or drug treatments in vivo.
- Characterize patient samples (e.g. tumor vs. healthy cells).
Disadvantages: The drawbacks to primary cells rely on the type of tissue and cell type you want to study. When studying primary cells, it can be challenging to:
- Isolate adequate cell numbers, either due to cell sensitivity during isolation (e.g. FACS) or low abundance in tissues.
- Obtain cells with high viability – primary cells often require careful handling to prevent lysis.
- Expand in culture. Their ability to expand in culture is usually limited to a few passages, requiring careful planning and optimization of experimental timelines.
- Generate consistent results, especially from bulk tissues containing many distinct cell types.
Figure 3: H1299 non-small cell lung carcinoma cells in culture. Image by Liz Albertorio-Sáez M.Sc.
Stem cells
Examples: Induced pluripotent stem cells (iPSCs), mesenchymal stem cells, and embryonic stem cells.
Advantages: The ability to grow and culture pluripotent and adult stem cell populations provides a wealth of opportunities to:
- Study epigenetic changes during cell development, differentiation, and disease progression.
- Generate rare cell types for functional analysis and therapeutic development.
- Develop patient-specific cell populations for personalized medicine applications.
- Study cell growth and differentiation using two-dimensional and/or organoid cultures.
Disadvantages: Despite their promise in cell and gene therapy research and developmental biology, working with stem cells can be challenging in many respects:
- Stem cell culture requires high-level expertise, and even then, expanding some stem cell populations is difficult.
- Culturing stem cells requires multiple quality control measures and precise monitoring, translating into expensive, time-consuming efforts.
- Differentiation protocols can vary between researchers, introducing unexpected biases and variations.