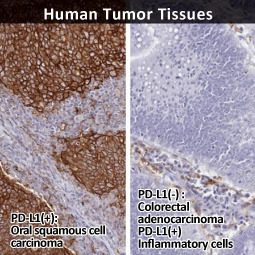
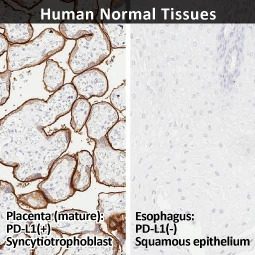
Inhibition of the PD-L1/PD-1 interaction has become a major clinical strategy against a number of solid malignancies. However, a durable response is obtained in a minority of patients, due in part to an incomplete understanding of the regulatory processes controlling PD-L1 expression in the tumor microenvironment. Thus, expanding our knowledge of these mechanisms will serve to not only enhance present anti-PD-L1/PD-1 clinical protocols but also potentially identify novel approaches.
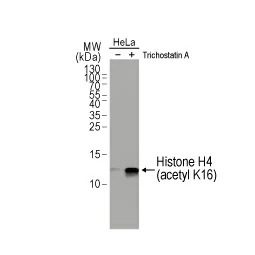
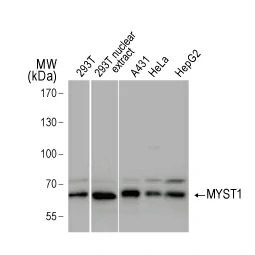
In their new study, Wu et al. examined the molecular features underlying interferon-gamma (IFNγ)-stimulated upregulation of PD-L1 (1). The authors used whole-genome CRISPR-mediated knockout screening to reveal that the histone acetyltransferase KAT8 (MYST1) forms a biomolecular condensate with the PD-L1-inducing IFNγ pathway transcription factor IRF1. Condensate formation, which is dependent on both specific and promiscuous interactions between KAT8 and IRF1, results in K78 acetylation of IRF1 by KAT8 (which enhances the former’s DNA binding) synergizing with H4K16 acetylation at the CD274 (PD-L1) promoter to increase PD-L1 mRNA expression. The researchers also identified an inhibitory peptide that disrupts the KAT8-IRF1 condensate leading to decreased PD-L1 expression and augmented antitumor immune responses.
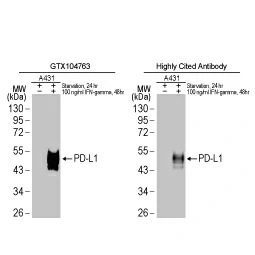
GeneTex offers an extensive catalog of quality antibodies and reagents for cancer research, including the polyclonal PD-L1 antibody (GTX104763) cited in the Wu et al. study. In addition, GeneTex is excited to introduce its extensively validated recombinant rabbit monoclonal PD-L1 antibody [HL1041] (GTX635975).