Comprehensive Guide to Performing Double Immunofluorescence
 |
 |
In attempting to characterize cellular and molecular processes, it often becomes necessary to label — either through immunofluorescence or immunohistochemistry — two or more targets in the same sample. This type of labeling provides insight into the localization of independent targets within cells or tissue and can help determine whether two or more targets co-localize under different experimental conditions.
However, cross-reactivity and non-specific binding of antibodies makes double-IF challenging for many researchers, especially when using primary antibodies with a high degree of immunogen sequence homology.
In this post, we present several techniques for double-IF staining using various combinations of primary antibodies, as well as some general guidelines for optimizing and troubleshooting such experiments.
Topics:
- Double staining: IF vs. IHC
- Simultaneous Incubation, Primary Antibodies from Different Host Species
- Alternate Detection Methods, Primary Antibodies from Different Species
- Staining high-abundance targets with conjugated primaries
- Primary Antibodies from Same Species
- Serial Sections
Double staining: IF vs. IHC
While double IHC labeling is possible under certain circumstances, we most often recommend immunofluorescence as the detection method. The wider range of available fluorophores makes it easier to select conjugations with little to no signal interference. In addition, the combined, overlapping fluorescence produced by co-localized targets is easier to characterize than overlapping IHC staining.
The excitation and emission spectra must be carefully considered when choosing fluorophores. Overlapping excitation ranges may result in false positive results as both fluorophores will be activated by the same light wavelength. In the same regard, overlapping emission spectra can make it difficult to differentiate unique probes.
Simultaneous Incubation, Primaries from Different Host Species
Primary antibodies from different species can be incubated simultaneously if the conditions for IF staining have been optimized independently. A mixture of the two primaries at their respective dilutions can be applied directly to paraffin-embedded or frozen sections overnight at 4°C with gentle agitation.
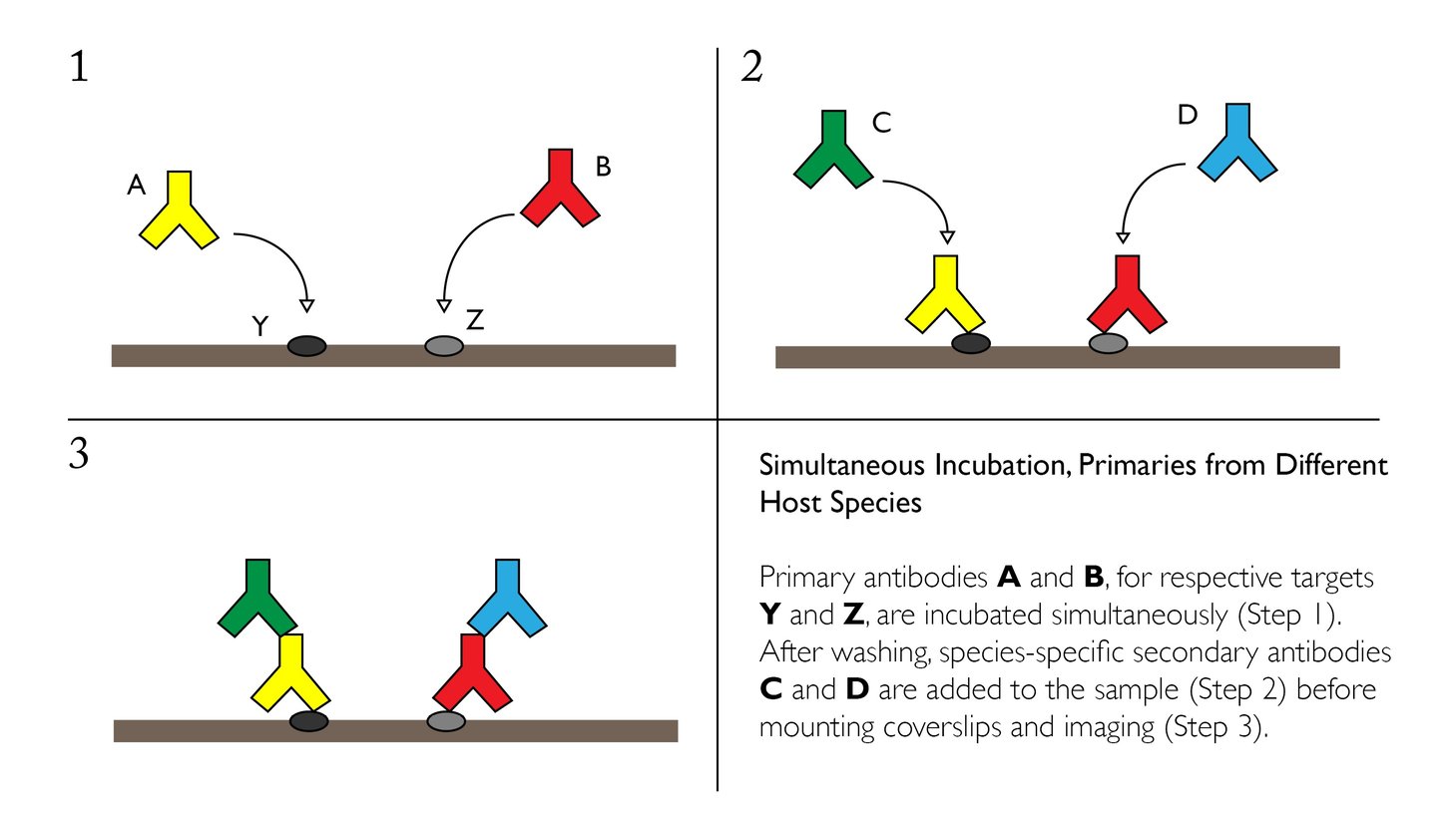
Advantages:
- Easy and relatively quick to perform with accessible reagents and materials
- Normal to high sensitivity for many targets with limited cross-reactivity
- Ability to use conjugated secondaries, which are usually offered with a wide selection of fluorophores and other probes
Disadvantages:
- Once antibodies are mixed, they must undergo the identical treatment conditions (i.e. incubation time, temperature, etc.), which limits flexibility
- Primary antibodies must be available from different species
- May result in uneven staining when targets vary greatly in their abundance
General Protocol:
- Perform fixation and permeabilization as necessary according to standard protocols
- Wash 3 times with cold TBS-t for 5 minutes at RT
- Block with 5% serum or BSA for 2 hours at RT
- Create mixture of primaries at the optimized dilution for each antibody in 1% blocking solution in TBS or preferred buffer
- Incubate overnight at 4°C with gentle agitation
- Remove primaries and wash 3 times with cold PBS for 5 minutes
- Incubate with mixture of fluorescently labeled secondaries for 1 hr at RT in the dark.
- Secondaries can be raised in the same species (e.g. goat-anti-mouse and goat-anti-rabbit), but must 1) be reactive to the respective primary antibodies and 2) be conjugated to fluorophores with distinct emission spectra.
- Remove secondaries and wash 3 times with cold PBS for 5 minutes
- Perform DAPI or alternate counterstain if desired
- Wash 3 times with cold PBS for 5 minutes
- Mount coverslips and store in the dark at 4°C until ready to image
Simultaneous Incubation using Biotin Conjugation, Primaries from Different Species
If cross reactivity of your secondary antibodies presents an issue when using simultaneous incubation of primaries, one alternative is to incorporate Avidin-Binding Complex or similar systems on one of the targets. This strategy may also be useful if one of the desired targets is in low abundance.
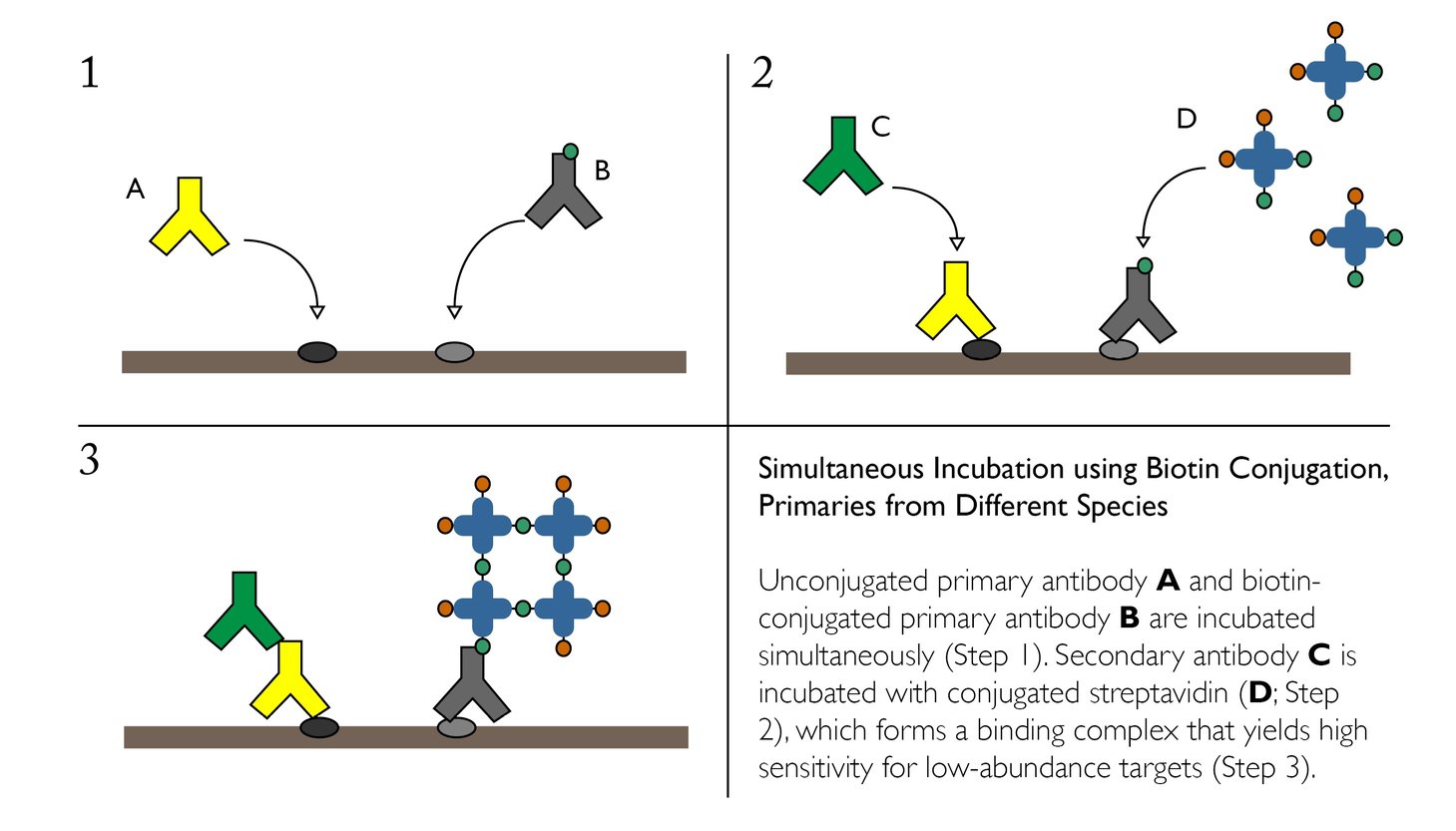
Advantages:
- Ability to detect multiple targets, even when one is low-abundance
- Only calls for one secondary antibody
- Quick and easy — does not increase the number of incubation steps
- Difficulty associated with simultaneously using two detection methods — a secondary antibody and the streptavidin complex
- May require more extensive optimization
- Requires the availability of biotin-conjugated primary of interest
General Protocol:
- Perform fixation and permeabilization as necessary according to standard protocols
- Wash 3 times with cold TBS-t for 5 minutes at RT
- Block with 5% serum or BSA for 2 hours at RT
- Mix biotin-conjugated primary and unconjugated primary at the optimized dilution for each antibody in 1% blocking solution in TBS or preferred buffer. It is recommended that biotin-conjugation be used to detect the protein with expected lower abundance, as ABC staining will increase sensitivity.
- Incubate overnight at 4°C with gentle agitation
- Remove primaries and wash 3 times with cold PBS for 5 minutes
- Incubate with mixture of 1) fluorescently labeled avidin or streptavidin (e.g. FITC-streptavidin) and 2) fluorescently labeled secondary for the unconjugated primary for 1 hr at RT in the dark.
- Remove secondaries and wash 3 times with cold PBS for 5 minutes
- Perform DAPI or alternate counterstain if desired
- Wash 3 times with cold PBS for 5 minutes
- Mount coverslips and store in the dark at 4°C until ready to image
Staining High-Abundance Targets with Conjugated Primaries
Cross-reactivity can be mitigated in some cases through the use of one or more conjugated primary antibodies. Since these products are directly linked to a fluorophore or other reporter molecule, it eliminates the need for another secondary antibody, reducing the risk for non-specific labeling.
Conjugated primary antibodies are best suited for high-abundance targets, and should be applied after any unconjugated/secondary pairs.
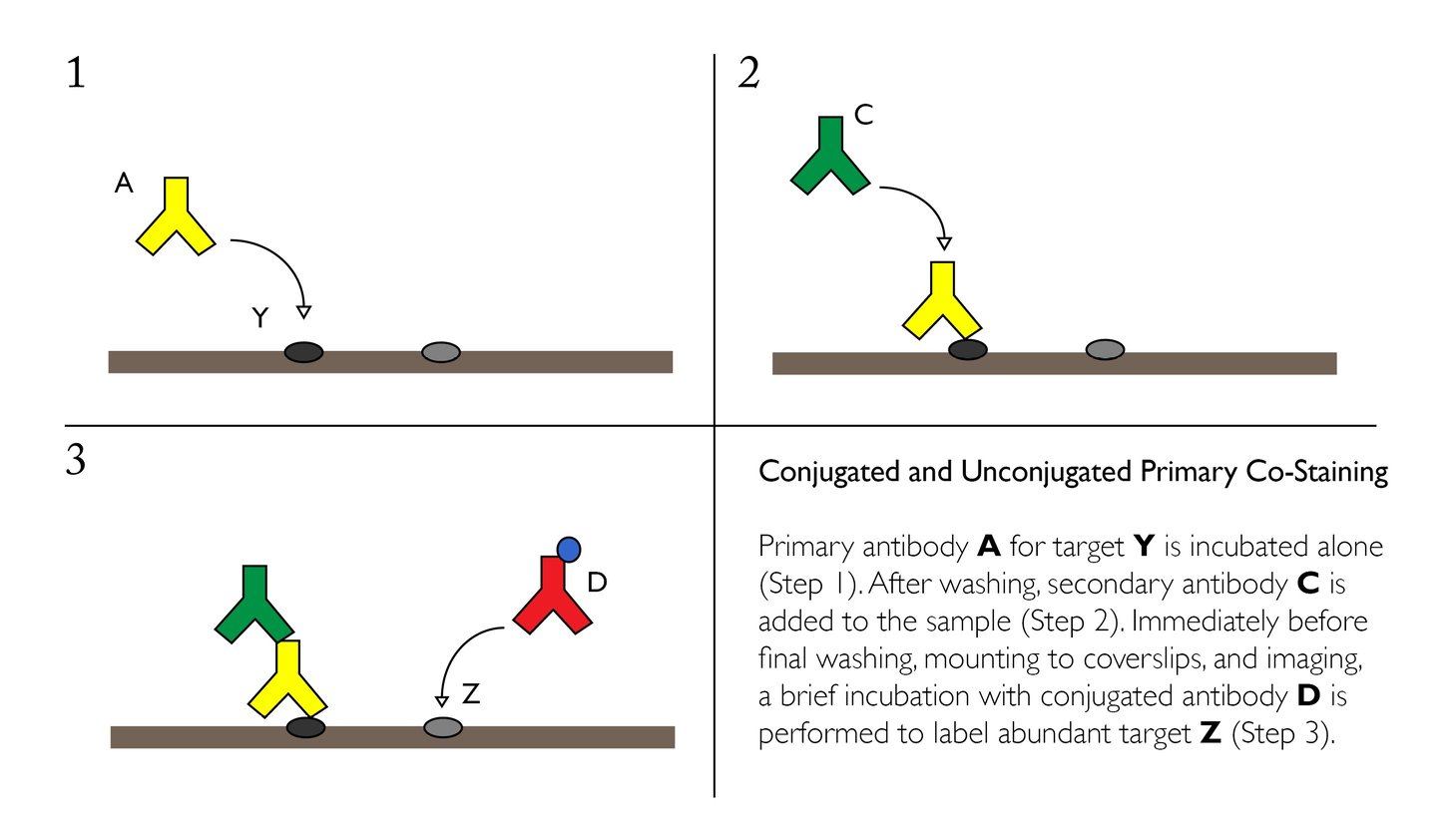
Advantages:
- Fewer secondary antibodies used, reducing the potential for species-to-species cross reactivity
- Conjugated antibodies require only a single incubation step
- Conjugated antibodies are ideal for labeling high-abundance proteins
Disadvantages:
- Conjugation site may alter or reduce antigen binding efficiency
- Potential cross-reactivity with the unbound antigen-binding site on the secondary antibody. Can be controlled through the addition of a suitable isotype control to block open sites before adding the conjugated primary
- Limited to emission spectra available for the desired primary antibody — less flexibility than choosing a secondary
General Protocol:
- Perform fixation and permeabilization as necessary according to standard protocols
- Wash 3 times with cold TBS-t for 5 minutes at RT
- Block with 5% serum or BSA for 2 hours at RT
- Incubate with unconjugated primary overnight at 4°C with gentle agitation
- Remove primary and wash 3 times with cold PBS for 5 minutes
- Incubate with fluorescently labeled secondary antibody for 1 hr at RT in the dark.
- Remove secondary antibody and wash 3 times with cold PBS for 5 minutes
- Incubate with conjugated primary antibody for 30 min to 1 hr at RT
- Wash 3 times with cold PBS for 5 minutes
- Mount coverslips and store in the dark at 4°C until ready to image
Primaries from Same Species
Application of primary antibodies from the same species is not recommended. Secondary antibodies are extremely likely to cross react, resulting in non-specific labeling.
If you decide you must use two primary antibodies from the same species — likely due to limited availability of antibodies targeting the necessary proteins — we recommend that you contact our Scientific Support team at technical@stratech.co.uk for a custom assessment of your experimental conditions and protocol.
Serial sections
One final alternative to double immunofluorescence is to label serial sections independently and attempt to interpret localization by examining closely related tissue. This allows the greatest range of flexibility for choosing antibodies and pretreatment conditions, as each tissue section can be treated under optimized protocols. However, serial sections will not provide proof of co-localization, since the fluorescent labels are not able to interact within the same tissue structure.