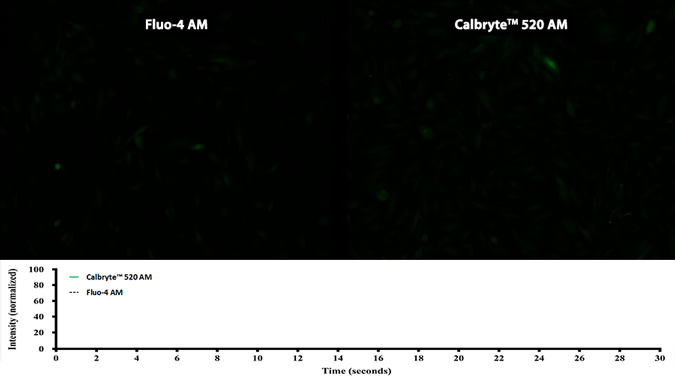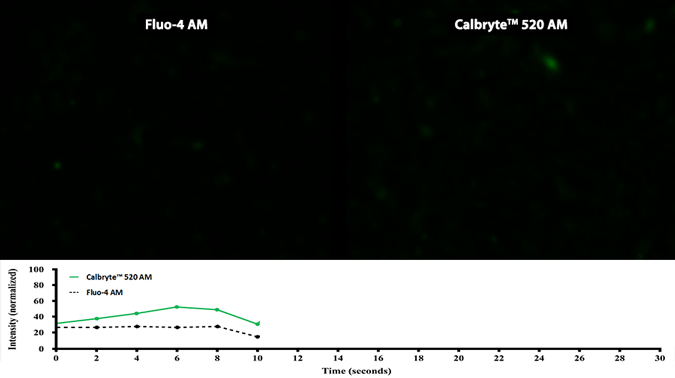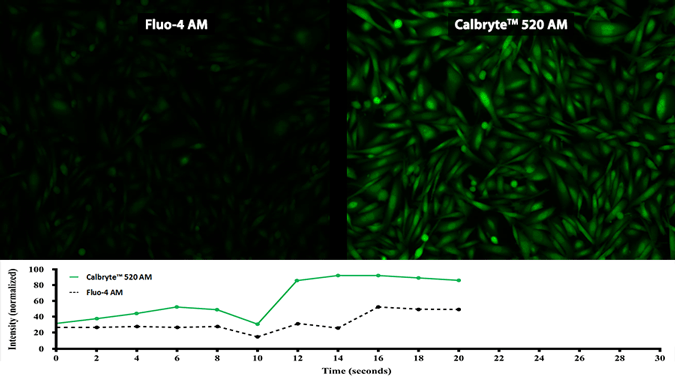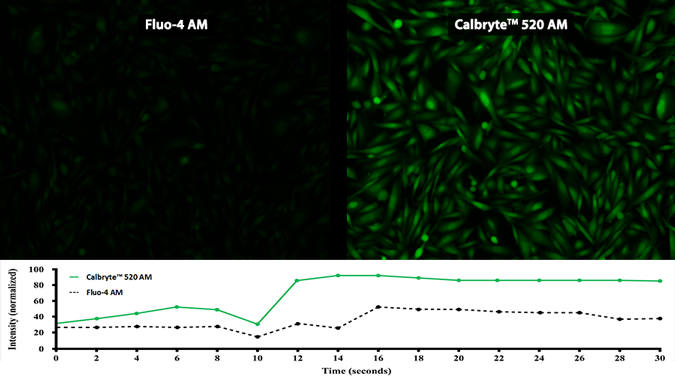
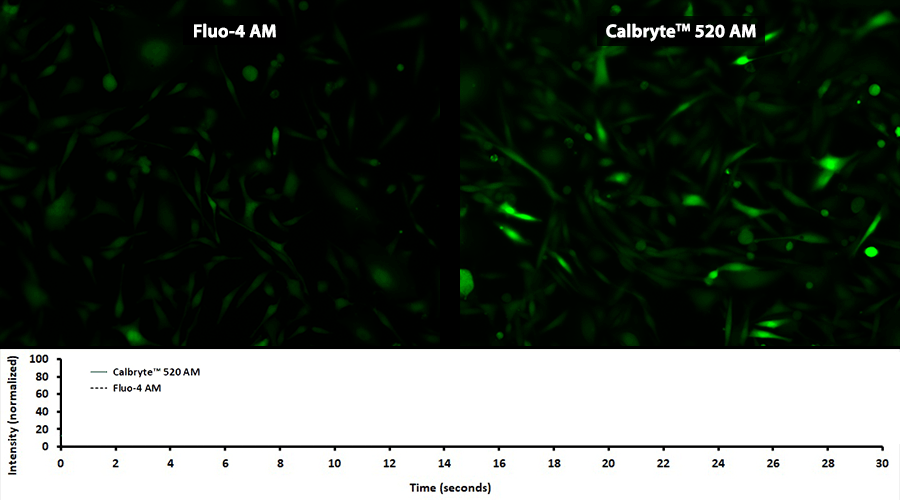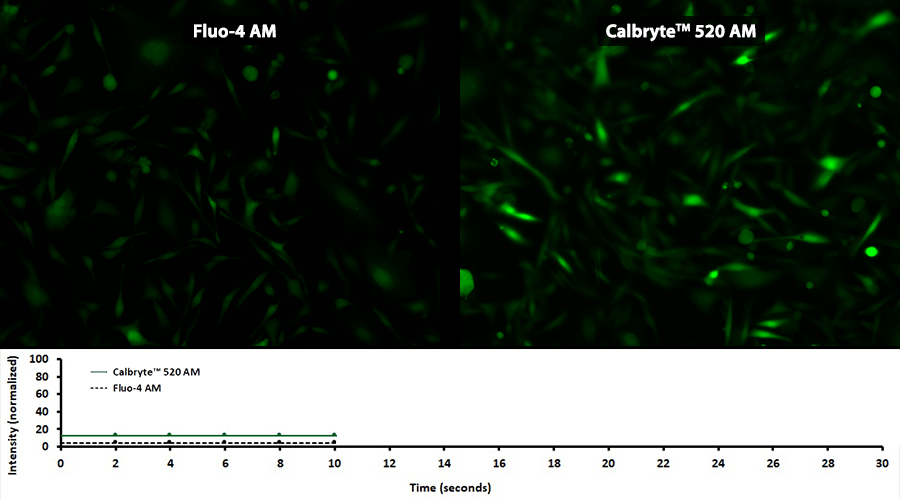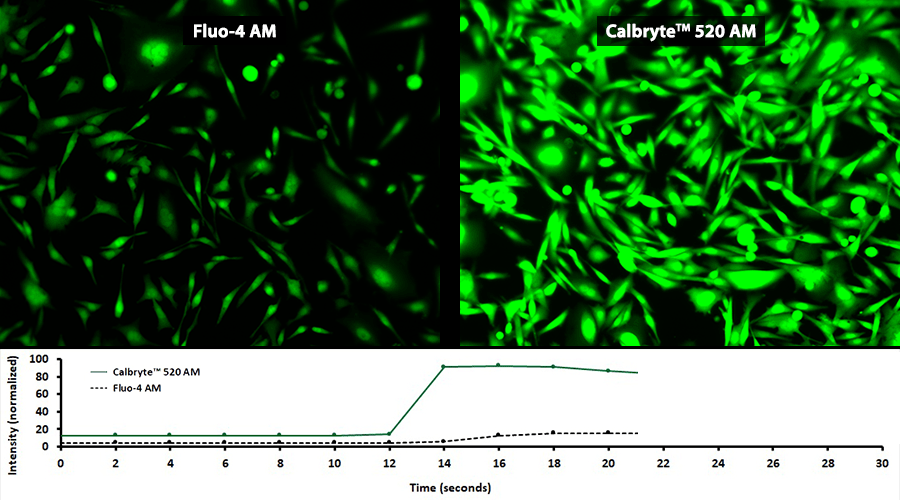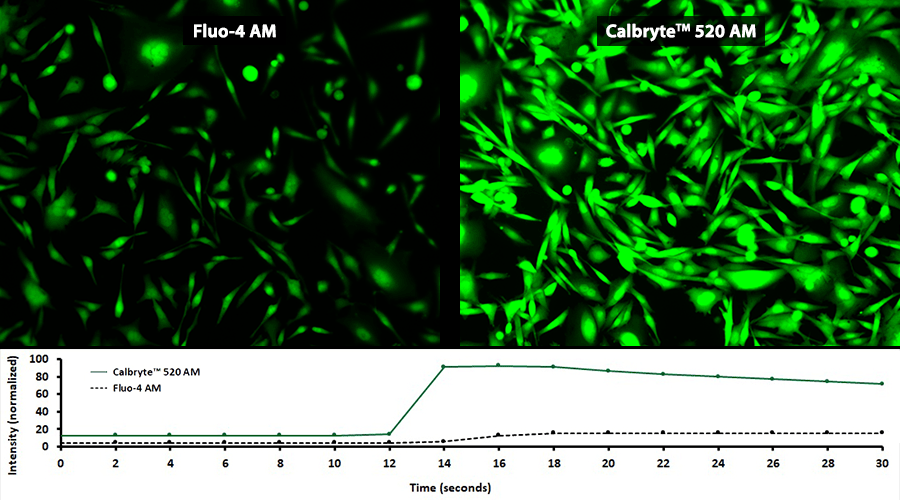
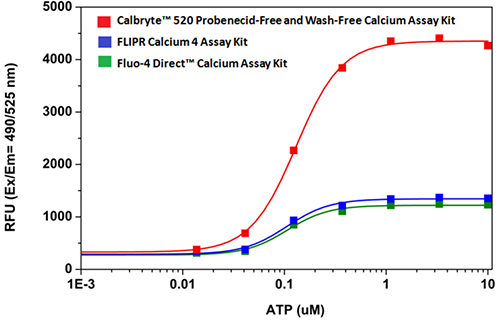
Quantifying Cytosolic Calcium
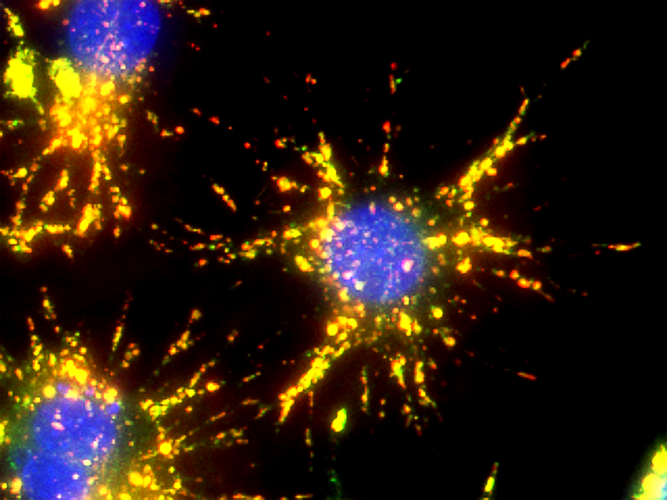
Calcium is a ubiquitous messenger impacting a number of signal transduction pathways including calcium-induced changes in protein confirmation, the regulation of cytosolic and organelle calcium levels, the highly localized nature of calcium-mediated signal transduction and its specific roles in excitability, exocytosis, motility, apoptosis, and transcription. These pathways regulate many key cellular processes including neurotransmission, cell proliferation, and muscle contraction. Under normal conditions with respect to the extracellular fluid, the resting cytoplasmic concentration of calcium is relatively low, at or below 100 nM. Calcium signaling is activated either through the release of calcium ions from intracellular stores, or by an influx of extracellular calcium through plasma membrane ion channels. Stimulated cells can increase cytoplasmic calcium concentration up to 500 to 1000 nM.
Fluorescent AM Ester Derivatives for Live Cell Calcium Imaging
Fluorescent indicators that show spectral responses upon binding calcium have enabled researchers to investigate changes in intracellular free calcium concentrations by using fluorescence microscopy, flow cytometry, fluorescence spectroscopy and fluorescence microplate readers. To facilitate detecting intracellular calcium mobilization and promote passive diffusion, acetoxymethyl ester (AM ester) functional groups are attached to the core structure of calcium indicators. This increases the indicator’s hydrophobicity allowing it to easily penetrate intact plasma membranes, and enhances indicator sensitivity by minimizing non-specific background fluorescence because AM ester conjugated indicators are essentially non-fluorescent and inactive.
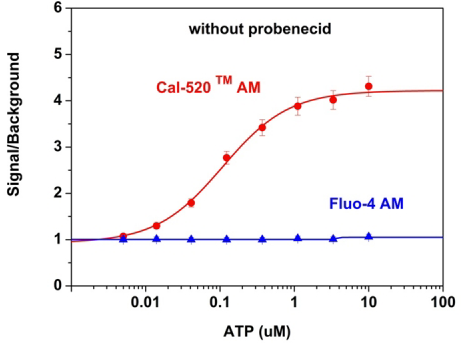
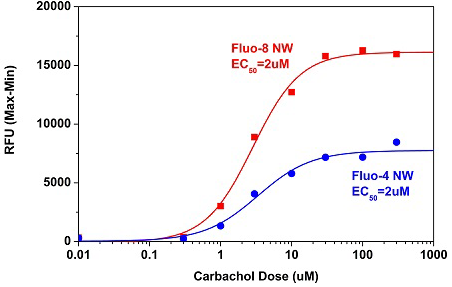
Activity is initiated once the probe enters a live cell and only after intracellular esterases remove the AM ester functional groups. Once activated, probes detect calcium through chelation and respond by generating a fluorescence signal relative to the concentration of calcium. Indicators such as Fluo-8®, AM (Cat No. 21080), Cal-520®, AM (Cat No. 21131) and Calbryte™ 520, AM (Cat No. 20653) have been extensively used in calcium imaging, calcium flux assays and cell-based high throughput screening (HTS) assays for drug discovery. Calcium indicators are compatible with use in flow cytometry, fluorescence microscopy, and the fluorescence microplate platform. Besides offering fluorescent and luminescent calcium indicators, we also provide several non-fluorescent calcium signaling molecules and chelators for measuring and manipulating intracellular and extracellular calcium.
Single-Wavelength Calcium Indicators
Single-wavelength calcium indicators are widely used to monitor calcium mobilization, image the spatial dynamics of calcium signaling, and in HTS cell-based pharmacological screening of agonist-stimulated and antagonist-inhibited signaling through GPCRs. These indicators exhibit significant calcium-dependent changes in fluorescence intensity at saturating calcium levels without shifting either their absorbance or emission wavelengths. We’ve developed a variety of novel calcium indicators, including the Fluo-8® series, the Cal-520® series and the Calbryte™ series, designed to trace calcium mobilization with the brightest fluorescent signals and a range of wavelength and affinity options to satisfy many applications.
Key features of single-wavelength calcium indicators
- Minimal background fluorescence at resting calcium levels
- Improved signal-to-background ratios
- Greatly enhanced cellular retention
- Cell-permeant formats for live cell calcium analysis (compatible with imaging, flow cytometry and microplate assays, such as HTS)
- Cell-impermeant formats for loading by microinjection, patch pipette, or pinocytic loading
- Ultraviolet (UV), violet and visible light-excitable formats
- Low-, medium- and high-affinity formats provides a sensitivity range to satisfy many applications