Nicotinamide adenine dinucleotides are abundant soluble cofactors that undergo reversible oxidation and reduction in major metabolic pathways. In cells they are present in oxidized and reduced states as their unphosphorylated (NAD and NADH) and phosphorylated (NADP and NADPH) forms. These dinucleotides work in pairs, and each pair has distinct functions. They have become a point of focus in cancer research because, as metabolites, they can tie metabolic pathways to transcriptional control, epigenetics and cell signaling as cells switch from a normal metabolism to a cancer cell (proliferative) metabolism.
NAD/NADH
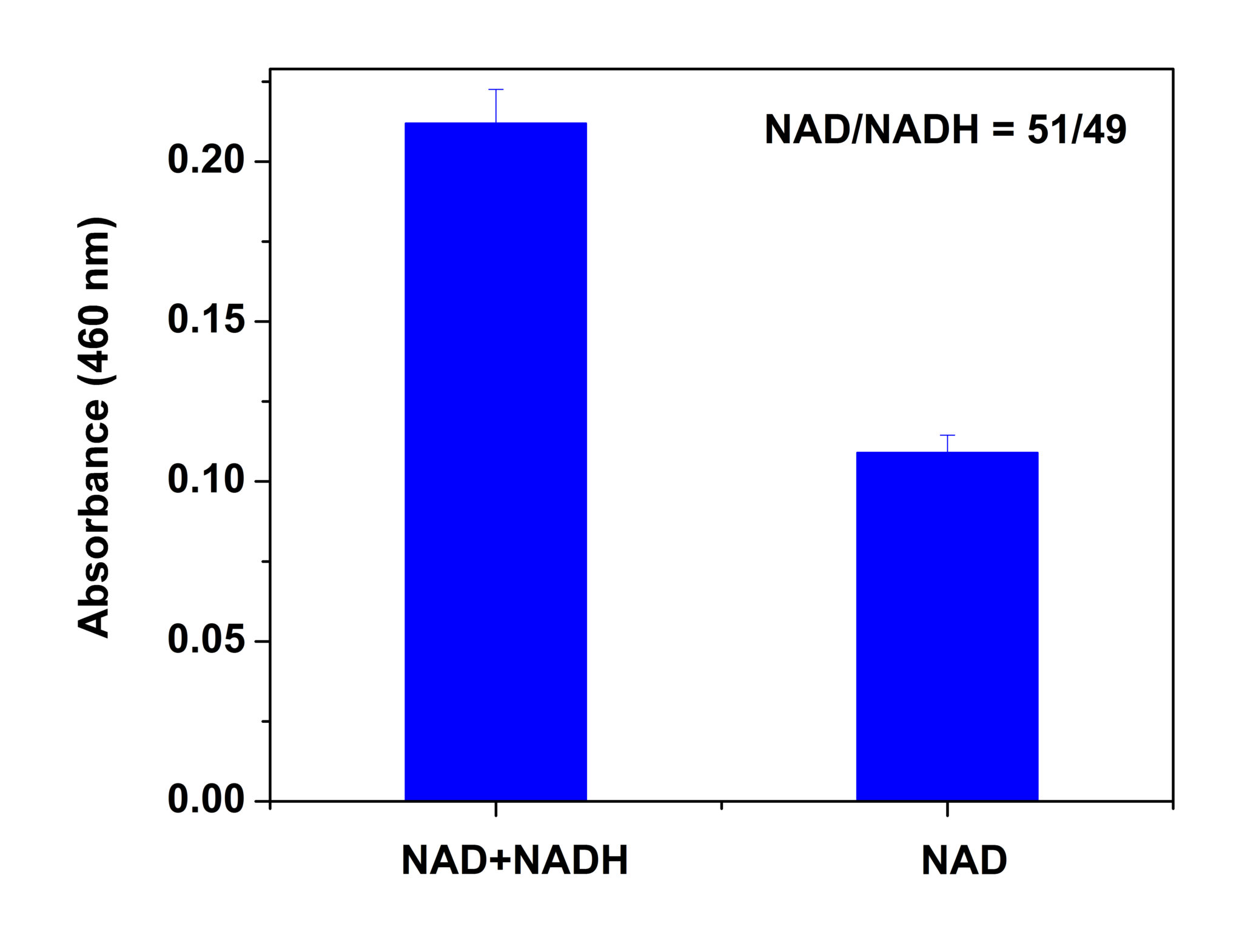
NAD is most often associated with catabolic pathways (glycolysis and oxidative phosphorylation); however its role in the cell involves more than energy metabolism. NAD is an important substrate in several signaling pathways and is involved in the epigenetic control of gene expression. It is the substrate used for ADP ribosylation and has roles in DNA repair , signaling regulation and signaling. The ratio of NAD/NADH influences the activity of many enzymes, especially the glycolytic enzymes.
NADP/NADPH
NADP/NADPH is the least abundant of the nicotinamide adenine dinucleotide pairs. NADPH is associated with biosynthesis of macromolecules, providing the reducing power necessary for those synthetic reactions. Increased biosynthesis is characteristic of rapidly proliferating cells, such as cancer cells; because of this NADPH is considered a key molecule produced as a result of cancer metabolism. It also has a role in responding to the buildup of Reactive Oxygen Species (ROS).
Monitoring the changes in intracellular levels of these important target-independent metabolites can allow us to better understand the link between altered metabolism and various diseases. Rapid and easy-to-use assays for key metabolites can facilitate these studies.