Reactive oxygen species (ROS) are chemically reactive, oxygen-containing molecules that are naturally produced as a byproduct of cellular metabolism. Under physiological conditions, ROS levels are carefully regulated, where they operate as messengers in normal cell signal transduction, cell cycling, gene expression and homeostasis.
When generated in excess, ROS has the potential to cause a number of deleterious events. At high concentrations, ROS induces oxidative stress in cells resulting in subsequent damage of cellular macromolecules such as nucleic acids, membrane lipids and cellular proteins. Oxidative damage of these biomolecules can trigger cell apoptosis, is associated with aging, and has been implicated in the pathogenesis of a variety of diseases, cancers and neurodegenerative disorders, as well as, diabetes and inflammation.
Because of its damaging effects, cells have several carefully regulated systems for managing excess ROS. The most well studied system is the glutathione-ascorbate cycle, which detoxifies H2O2 into H2O, using NADH and NADPH as electron donors. Other systems include enzymes such as superoxide dismutase, which catalyzes the dismutation of the superoxide anion (O2–) into O2 or H2O2, and catalase, which catalyzes the decomposition of H2O2 into H2O and O2.
Types of ROS
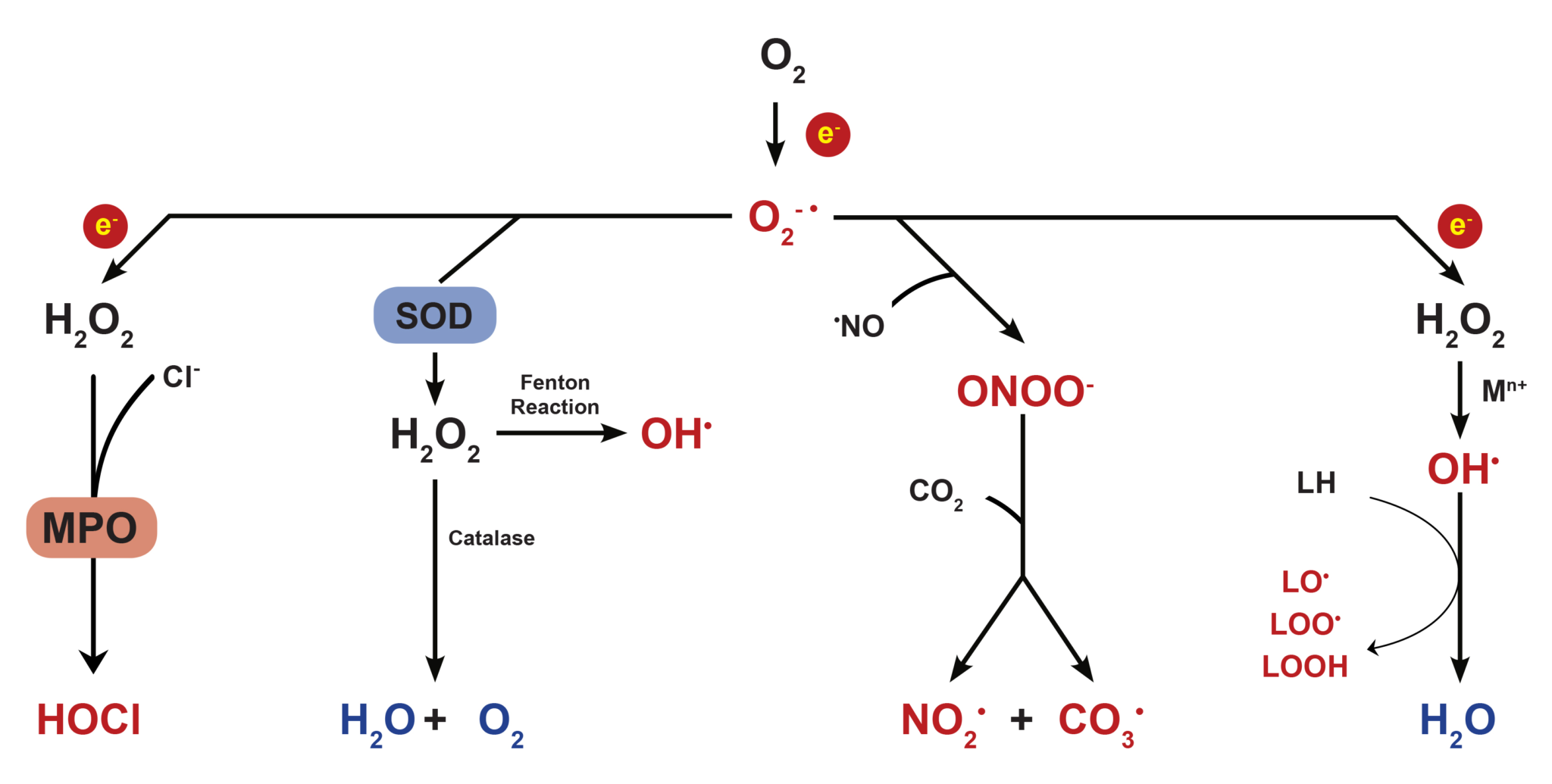
Most cellular ROS are generated endogenously as byproducts of mitochondrial oxidative phosphorylation, or formed as intermediates of oxidoreductase enzymes and metal catalyzed oxidation. Since oxygen atoms contain two unpaired electrons in separate orbits of its outer electron shell, it is susceptible to radical formation. The sequential reduction of oxygen through the addition of electrons leads to the formation of a number of ROS including superoxide anion (O2–), hydrogen peroxide (H2O2), hydroxyl radical (•OH), hypochlorous acid (HOCl), peroxynitrite anion (ONOO–), and nitric oxide (NO).
Figure 1. Reduction of oxygen and its byproducts.
Hydrogen Peroxide
Hydrogen peroxide (H2O2) is a reactive oxygen metabolic byproduct that serves as a key regulator for a number of oxidative stress related states. It is involved in a number of biological events that have been linked to asthma, atherosclerosis, diabetic vasculopathy, osteoporosis, a neurodegeneration and Down’s syndrome. Perhaps the most intriguing aspect of H2O2 biology is the recent report that antibodies have the capacity to convert molecular oxygen into hydrogen peroxide to contribute to the normal recognition and destruction processes of the immune system. Measurement of this reactive species will help to determine how oxidative stress modulates varied intracellular pathways.
Intracellular Hydrogen Peroxide Probes
| |
DCFH-DA |
Dihydrorhodamine 123 |
OxiVision™ Blue |
OxiVision™ Green |
| Principle |
Esterases cleave off diacetate group to yield DCFH which is oxidized by H2O2 to DCF and fluoresces green upon excitation |
Oxidation by H2O2 yields rhodamine 123, which fluoresces blue upon excitation |
OxiVision™ probes are oxidized by intracellular H2O2. Generates fluorescence upon excitation. |
| Ex/Em (nm) |
504/525 |
507/527 |
405/450 |
497/516 |
| Filter Set |
FITC |
FITC |
DAPI |
FITC |
| Live Cells |
Yes |
| Imaging |
Yes |
| HCS |
Yes |
| Flow Cytometry |
Yes |
| Unit Size |
25 mg |
10 mg |
Kit (100 Tests) |
Kit (100 Tests) |
| Cat No. |
15204 |
15206 |
11504 (Imaging)
11505 (Flow) |
11503 (Imaging)
11506 (Flow) |
Hydroxyl Radical
The detection of intracellular hydroxyl radical (•OH) is of central importance to the understanding of proper cellular redox regulation and the impact of its dysregulation on various pathologies. The hydroxyl radical is one of the reactive oxygen species (ROS) that are highly reactive with other molecules to achieve stability. In general, hydroxyl radicals are considered to be a harmful byproduct of oxidative metabolism, which can cause molecular damage in living systems. It shows an average lifetime of 10-9 nanoseconds and can react with nearly every biomolecule, such as nuclear DNA, mitochondrial DNA, proteins and membrane lipids.
Hydroxyl Radical Detection
- Intracellular •OH detection for live cells
Table 1. Intracellular ROS Selection Guide
|
ROS Species
|
ROS Brite™ 570
|
ROS Brite™ 670
|
ROS Brite™ 700
|
ROS Brite™ HDCF
|
Amplite™ ROS Green
|
Amplite™ ROS Red
|
| Hydrogen Peroxide (H2O2) |
+ |
+ |
+ |
+++ |
+++ |
+++ |
| Hydroxyl radical (•OH) |
++ |
++ |
++ |
+ |
+ |
+ |
| Tert-butyl-hyrdoperoxide (TBHP) |
+ |
+ |
+ |
+ |
+ |
+ |
| Hypochlorous acid (HOCl) |
– |
+ |
++ |
– |
+ |
– |
| Superoxide anion (O2•-) |
+ |
++ |
++ |
– |
– |
– |
| Nitric Oxide (NO) |
– |
– |
– |
– |
– |
– |
| Peroxynitrite anion (ONOO–) |
– |
– |
– |
– |
– |
– |
| Cat No. |
22902 |
22903 |
16004 |
16053 |
|
22901 |
Table 2. ROS-Selective Probes and Assay Kits
|
ROS Species
|
OxiVision™ Green
|
OxiVision™ Blue
|
MitoROS™ 520
|
MitoROS™ 580
|
MitoROS™ OH580
|
| Hydrogen Peroxide (H2O2) |
+++ |
+++ |
– |
– |
– |
| Hydroxyl radical (•OH) |
– |
– |
– |
– |
+++ |
| Tert-butyl-hyrdoperoxide (TBHP) |
– |
– |
– |
– |
– |
| Hypochlorous acid (HOCl) |
– |
– |
– |
– |
– |
| Superoxide anion (O2•-) |
– |
– |
+++ |
+++ |
– |
| Nitric Oxide (NO) |
– |
– |
– |
– |
– |
| Peroxynitrite anion (ONOO–) |
– |
– |
– |
– |
– |
| Cat No. |
|
|
16060 |
|
|
Additional Resources
Table 3. ROS generation methods
| Reactive Oxygen Specie (ROS) |
ROS Generation Method |
| H2O2 |
100 µM of H2O2 |
| •O2- |
100 µM of KO2 |
| ¹O2 |
100 µM of 3-(1,4-dihydro-1,4-epidioxy-1-naphthyl)propionic acid |
| -OCL |
3 µM (final) of -OCl |
| • OH |
100 µM of ferrous perchlorate (II) and 1 mM of H2O2 |
| ONOO- |
3 µM (final) of ONOO- |
| NO |
100 µM of 1-hydroxy-2-oxo-3-(3-aminopropyl)-3-methyl-1-triazene |
| ROO• |
100 µM of 2,2?-azobis(2-amidinopropane), dihydrochloride (AAPH) |
| Auto-oxidation |
2.5 hours of exposure to a fluorescent light source |