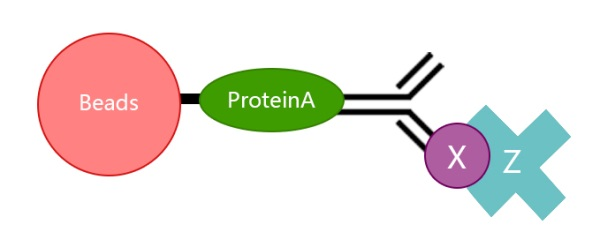
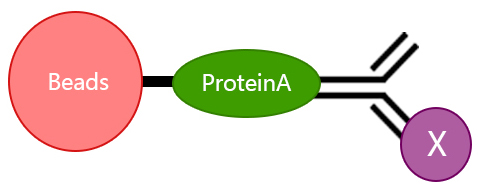
3.Do IP antibodies and WB antibodies need different provenances?
It is best to choose antibodies from two different provenances. The problem of choosing the same antibody source lies in that in the step of protein denaturation before WB, the mercaptoethanol contained in the sample buffer will destroy the disulfide bond between the heavy chain and the light chain of the antibody, thus making the antibody become a heavy chain molecule 55KD and a light chain molecule 25KD. Assume that the antibody to your IP is rabbit-resistant, the antibody to WB made by the target protein is rabbit-resistant, and the molecular weight of the target protein is 55KD. It can be imagined that the position of 55KD is not only your target protein, but also the heavy chain IgG of IP antibody. As the amount of antibody used in IP is very large (1ug), the signal at 55KD will be very strong. If you use anti-rabbit secondary antibodies for color development, then the heavy chain of the ip antibody at 55KD and your target protein will overlap and be indistinguishable.
However, at this time, if the antibody of your target protein to WB is not rabbit-resistant, such as murine resistant, then it is necessary to use the second antibody of anti-mouse for color development, and the second antibody of anti-mouse cannot react with the IP antibody of rabbit resistant. Therefore, the heavy chain IgG of the IP antibody at 55KD is not chromogenic, and the reaction is your target protein.
4.What is input in co-immunoprecipitation? What is the significance of a negative coprecipitation control?
input is a positive control. In the immunocoprecipitation experiment, WB is directly taken from the cell lysate to verify that the target protein is indeed present in the cell lysate, namely, the positive control.
A negative control is used to eliminate the possibility of contamination, for example by detecting a protein in a rabbit cell. If no control is set for IgG, a non-specific protein is present in the operation, and the antibody is designed to interact with it, a positive result will occur. If you do a negative control, which is the lgG control, if you don’t have a positive control, that means you don’t have a nonspecific protein, and if you have a positive control, that means there’s a potential for nonspecific binding of the antibody, and you need to start the experiment again.
5.How to remove the influence of heavy and light chains in the Co-ip immunoprecipitation experiment?
a: 1. The source of IP primary antibody is the same as that of WB primary antibody; 2. When the source of two antibodies is inconsistent, the secondary antibody has cross-reaction: the use of IPkine secondary antibody can avoid the interference of light and heavy chains;
b: The abundance of target protein is low, and the exposure time of the instrument is prolonged, which leads to the appearance of stray bands in the IgG negative control. The amount of samples in the Input and IP lanes is increased, and the exposure time is reduced to prevent the interference of light and light chains (stray bands).
c: Excessive addition of negative control IgG and IP primary antibody: the amount of control IgG antibody is too large, which is easy to lead to the formation of stray bands. It is recommended to adjust the dosage of negative control IgG to less than 1ug.