Immunotherapy is an exciting strategy for the treatment of cancer, designed to re-activate and enhance anti-tumor immune response, thereby restoring tumor immune suppression (Waldman et al., 2020). Immune checkpoint inhibitors, such as the programmed death 1 (PD-1) receptor and its ligand programmed death ligand 1 (PD-L1) pathway, are promising therapeutic options that have shown clinical success (Zhuansun et al., 2016)).
Monoclonal antibodies are a valuable resource for cancer immunotherapy, and Absolute Antibody offers three recombinant anti-PD-1 clones (Table below).
Programmed cell death protein 1 (PD-1) is a checkpoint protein mainly expressed on activated T cells, natural killer cells and B cells. PD-L1 is one of its known ligands, which interacts with PD-1 on T cells preventing T-cell activation and proliferation, thus down-regulating the immune system (Zhou et al., 2019). The PD-1/PD-L1 pathway plays a key role in reducing the regulation of ineffective or harmful immune responses and maintaining immune tolerance, for instance, preventing autoimmune diseases. In the context of a tumor, however, these interactions suppress the host immune response, leading to the spreading of the tumor (Sharpe et al., 2017).
In normal conditions the T cells of the host recognize and destroy tumor cells. However, tumors can bypass immune surveillance by exploiting immune-escape mechanisms, such as the PD-1/PD-L1 pathway. PD-L1 is often overexpressed on the surface of the tumor cells and the interaction of PD-L1/PD-1 promotes T cell dysfunction, exhaustion, apoptosis and neutralization, inactivating the immune system and promoting cancer development and progression (Akinleye et al., 2019). Therefore, inhibition of the interaction between PD-1 and PD-L1, also called checkpoint blockade, has represented a therapeutic strategy for tumor immunotherapy.
Monoclonal antibodies blocking PD-1 and PD-L1 have been successfully employed as therapeutic agents for checkpoint blockade. For instance, the FDA has approved some anti-PD-1 and anti-PD-L1 antibodies, including pembrolizumab and nivolumab, which have shown clinical efficacy in many tumors. Although the results are promising, most patients do not show long-lasting remission, and the risk of adverse events is still relatively high (Liu et al., 2021). Therefore, further investigation into the PD-1 pathway mechanisms is necessary to improve their therapeutic success.
Recombinant Engineered Antibodies
The Absolute Antibody catalog offers three anti-PD-1 clones: RMP1-14, 29F.1A12 and J43. The anti-PD-1 clones are recombinantly produced to ensure batch-to-batch reproducibility and have been further engineered to better suit in vivo research in mice. They are part of our VivopureX™ collection, which consists of clones that have been engineered into mouse-anti-mouse recombinant versions to reduce immunogenicity in mouse models. Moreover, the Fc Silent™ format contains key point mutations that abrogate binding to Fc receptors, abolishing antibody directed cytotoxicity (ADCC) effector function of Fc receptors.
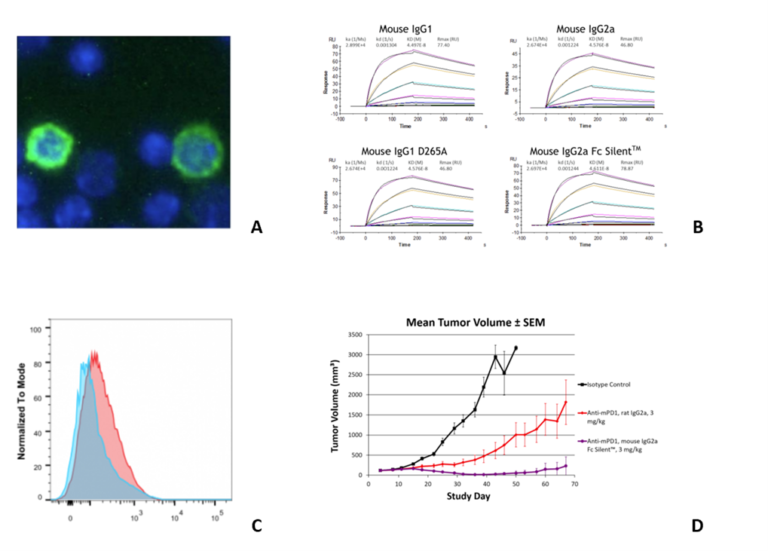
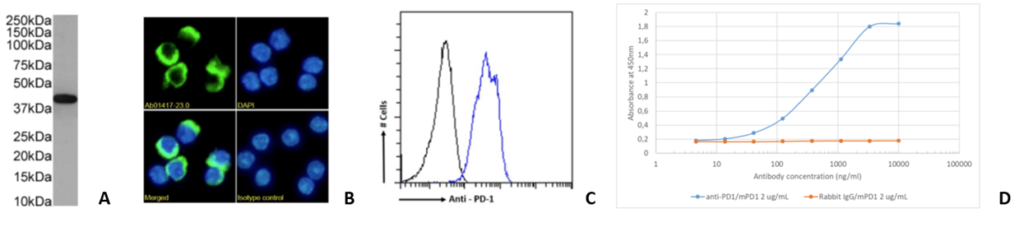
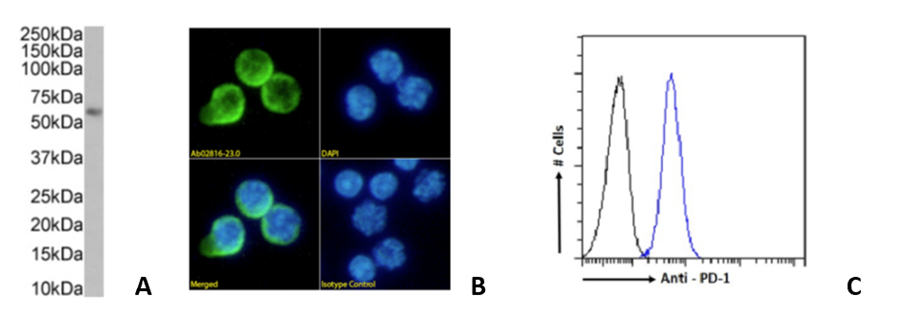
RMP1-14 and 29F.1A12 are originally rat IgG2a, while J43 is hamster IgG. The RMP1-14 clone is an in vivo blocking anti-PD-1 antibody. Further, our Fc Silent™ RMP-14 clone has been shown to have stronger anti-tumor activity than the original non-engineered rat antibody. The 29F.1A12 and J43 clones are in vivo PD-1 blocking and in vitro PD-1 neutralizing antibodies. Moreover, RMP1-14, 29F.1A12 and J43 are able to block the binding of PD-1 to PD-L1 and PD-L2. Figure 1 shows immunofluorescence, SPR, flow cytometry and in vivo analysis for clone RMP1-14 (Ab00813). Figure 2 shows Western blot, immunofluorescence, and flow cytometry analysis for clone J43 (Ab01417) and Figure 3 shows Western blot, immunofluorescence, and flow cytometry analysis for clone 29F.1A12 (Ab02816). The antigen, together with the reported applications and formats currently available on the website, are depicted in the table below.
Comparative studies between the clones have been carried out. A recent study reported that 29F.1A12 shows higher affinity and avidity for PD-1 than RMP1-14 (Bu et al., 2022). Cross-blocking experiments between the clones show that RMP1-14 does not interfere with detection of PD-1, J43 partially prevents PD-1 detection and 29F.1A12 strongly prevents PD-1 detection by the other two clones, respectively (Polesso et al., 2021).
Due to its higher affinity for PD-1, 29F.1A12 has been suggested as a better surrogate model for human anti-PD-1 therapeutics than RMP1-14. However, the use of moderate-affinity binders to improve targeting to highly expressing tissues is gaining popularity—especially with complex, multispecific biologics.
Thinking about murine bispecifics? Check out our murine bispecific antibodies and contact us to discuss your ideas and projects! We are dedicated to making your antibody possibilities a reality, and our Periodic Table of Antibodies (a collection of more than 180 antibody formats we have created) shows just how many possibilities exist for your experimental goals!
Interactions between PD-1 axis and the B7 family
The B7 family consists of seven structurally related, cell-surface protein ligands: CD80 (B7.1), CD86 (B7.2), inducible costimulator ligand (ICOS-L), PD-L1, PD-L2, B7-H3, and B7-H4. Members of the B7 family bind to receptors on lymphocytes that regulate immune responses. Interaction of B7 family members with costimulatory receptors augments immune responses, and interaction with coinhibitory receptors attenuates immune responses (Collins et al., 2005). As such, PD-1 is the receptor for PD-L1 and PD-L2 ligands. Recent studies reported that PD-L1 can also bind to CD80, inhibiting the PD-1 axis and consequently the T cell activation (Sugiura et al., 2019).
If your research involves the study of the interactions between PD-1 with members of the B7 family, review our VivopureX™ range of anti-mouse PD-L1 (10F.9G2 and 10F.9G2 Fc Silent™), PD-L2 (TY25 Fc Silent™) and CD80 (RM80 Fc Silent™) clones.
Please contact us if you are interested in anti-PD-1 antibodies, their applications, or their availability in different formats. If you’re interested in immune checkpoint inhibitor antibodies, find out more on how our recombinant antibodies engineered to facilitate in vivo research can help you in your preclinical research.