It is common for sample collection to occur days, weeks, or months before all downstream analysis is fully complete. Maintaining the integrity of samples for short- to long-term preservation and downstream analysis has long been a challenge for researchers.
Some may remember formaldehyde fixation from high school anatomy experiments – the distinct smell and warnings of its high toxicity certainly made an impact. Formaldehyde in aqueous solution is known as formalin. Specimen tissue is often formalin-fixed paraffin-embedded (FFPE) in order to preserve the tissue for transport and storage at room temperature prior to analysis. Like formaldehyde, formalin is still toxic, and FFPE samples are often difficult to use for downstream analysis due to molecular changes caused by the formalin.
Scientists have frozen samples for cell fixation as another means of preserving for quite some time, however this requires significantly more logistics than FFPE, as the sample would need to be fresh-frozen at the site of sample collection.
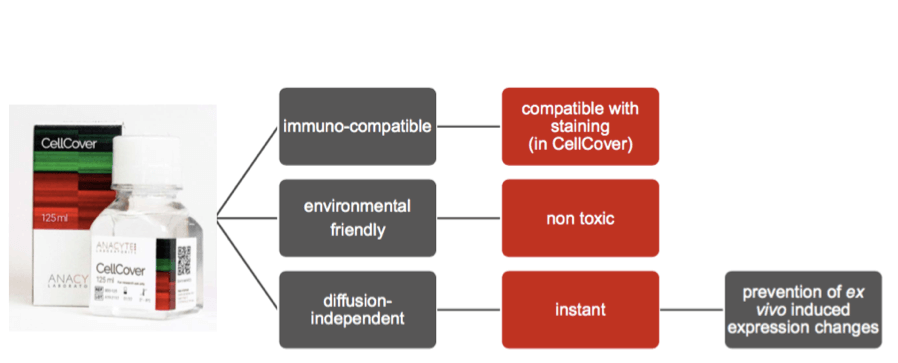
CellCover takes things one step further by essentially “liquid freezing” samples in the refrigerator (usually at 4°C), acting as a much more practical formaldehyde alternative and freezing alternative.