What blood sampling cards are available on the market today?
A number of specialised blood sampling cards exist, but only two types are pre-qualified by the United States Centers for Disease Control and Prevention (CDC) for use in NBS. These are cards comprising the Whatman 903 Specimen Collection Paper or the PerkinElmer 226 Specimen Collection Paper, which primarily serve the US and European markets, respectively.
To be approved for use in NBS, blood sampling cards must as a minimum conform to three major criteria as laid out by the US Clinical & Laboratory Standards Institute (CLSI):
- The applied blood must be absorbed into the card within a certain time period
- The blood must spread homogenously across the card, and…
- It must be demonstrable via isotopic labelling that the blood has spread homogenously through the card
Since Guthrie’s work in the 1960s, NBS has become routine in most parts of the world. Today, NBS programmes screen for congenital hearing loss, critical cardiac heart defects, and numerous metabolic, blood, endocrine, immunological, and other genetic disorders. These screening programmes are qualitative, and a yes/no answer in the heel-prick test shortly after birth will prompt follow up testing to confirm or rule out the suspected condition. Importantly, early detection facilitates timely treatment for better overall prognoses.
Ahlstrom Grade 222 and 320 filter papers are used in Capitainer®B10 and Capitainer®B50, respectively, for the accurate collection of 10 μL or 50 μL of capillary blood. These filter papers are thicker than those used in NBS, and they offer extremely high absorbency which enables a larger sample volume in a smaller disc diameter of 6 mm. As a side note, this diameter also allows the discs to fit neatly into the wells of 96-well plates for seamless pre-analytical processing, without any manual intervention. Finally, their uniform and smooth structure makes these cards ideal for applications that require accurate and reproducible sampling.
Traditional DBS microsampling is semi-quantitative at best
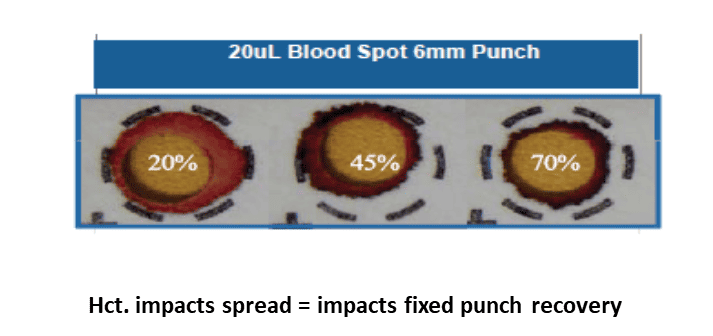
As mentioned above, NBS programmes are qualitative, and this is for several reasons. Firstly, it is standard practice to assume that a 3.2 mm diameter sub-punch equates to 3.1 μL of whole blood (2), but in reality, blood volumes can vary greatly across sub-punches of the same size. These variable and unknown blood volumes lead to analytical bias because a higher sampling volume usually leads to higher analyte recovery even across sub-punches of equal size. For example, previous studies have shown that, if a liquid blood sample containing phenylalanine at a concentration of 360 μmol/L is applied to filter paper in different volumes (10-100 μL), the mean concentration of the actual measured phenylalanine in the DBS samples (using a standard 3.2-mm sub-punch for analysis) would vary from 300 μmol/L in the 10 μL DBS to 400 μmol/L in the 100 μL DBS (3).
Other factors that render NBS blood sampling cards qualitative include the hematocrit effect, whereby the proportion of red blood cells in the blood influences blood viscosity and impedes homogenous spreading of blood throughout the card, as well as documented variation in analyte distribution, which was recently discussed in relation to PKU monitoring by Hogg et al., 2023 (2).
Therefore, while sufficient and ideal for the purpose of NBS and qualitative tests with yes/no answers, the blood sampling cards described so far are not suitable for applications that require quantification, e.g., TDM, viral load monitoring, and accurate monitoring of individuals with inborn errors of metabolism.
Volumetric microsampling allows quantitative analysis
The development of volumetric blood microsampling devices that consistently meter an exact known volume has made it possible to perform quantitative analyses based on dried blood microsamples. Capitainer’s devices are built upon proprietary microfluidic technology that makes it possible to meter a known blood volume to a pre-cut DBS disc, which serves as the sub-punch or sample for analyte extraction and analysis. In this way, we eliminate the biases associated with conventional blood sampling cards.
So far, our volumetric devices have already been explored with success in diverse applications including therapeutic drug monitoring, quantitative detection of biomarkers for cardiovascular disease, and large-scale serological surveillance, with several other investigations ongoing.