Exosomes Explained
How to use Exosomes
Exosomes Tools, from isolation to engineering
Exosomes – extracellular vesicles (EVs) 30 – 200 nm in size were once thought to be just a way for cells to offload waste. They have now been shown to function as important signal carriers and tissue reshapers via their cargos of DNA, RNA, lipids and proteins. They are intrinsic in both healthy and pathogenic processes including immunity, cancer, inflammation, CNS function. These recently discovered functions are bringing them centre stage in a rapidly expanding area of study in basic research, biomarker discovery and engineered tools for therapeutic molecule delivery.
Our all-encompassing suite of exosome tools supplied by System Biosciences (SBI) can open the door for your work into this exciting area with easy to use tools and services from exosome isolation to cutting edge exosome engineering and delivery tools. Their tools include reagents and kits that support all aspects of exosome research – covering isolation, detection and measurement, discovery (characterisation and analysis), and even exosome engineering.
| ISOLATION | DETECTION | DISCOVERY | ENGINEERING |
|---|---|---|---|
| ExoQuick® and ExoQuick-TC® | Exo-Flow™ FACS | Purified Exosomes | Exo-Glow™ Cargo Labeling |
| ExoQuick-CG® | Antibodies | SeraMir™ RNA Purification | Exo-Fect™ Transfection |
| Exo-Flow™ IP | ExoCheck Ab Array | XRNA Illumina® NGS Kits | XMIRs miRNA Uploading Vectors |
| Exo-FBS™ | ExoELISAs | XPEP Mass Spec Kits | XPack Protein Packaging Vectors |
| EV-Entry Reagent | EXOCET Enzymatic Assay | EV Shuttle Kits | XStamp Exosome Targeting Technology |
Isolation
Polymeric precipitation is a quick method for isolating exosomes that does not require ultracentrifugation, can be used with smaller sample volumes, and can be used to quickly process a large number of samples, making it suitable for use with clinical samples. ExoQuick products use this approach, with ExoQuick-TC optimised for recovering exosomes from tissue culture and urine (fluids with higher concentrations of exosomes) and standard ExoQuick for other biofluids where exosome c oncentrations tend to be lower. With ExoQuick, researchers can isolate exosomes from as little as 250 µl of sample. Recovery is quantitative and linear as volume scales up, and the quality and purity of exosomes is suitable for a wide number of downstream applications. Please be aware that Foetal Bovine serum (FBS), one of the most widely used serum supplements in cell culture contains bovine exosomes. Therefore, when isolating Exosomes from Cultured Cells, it is essential to use Foetal Bovine Serm (FBS) that has had the native exosomes removed. For this application Exosome depleted FBS is perfect.
oncentrations tend to be lower. With ExoQuick, researchers can isolate exosomes from as little as 250 µl of sample. Recovery is quantitative and linear as volume scales up, and the quality and purity of exosomes is suitable for a wide number of downstream applications. Please be aware that Foetal Bovine serum (FBS), one of the most widely used serum supplements in cell culture contains bovine exosomes. Therefore, when isolating Exosomes from Cultured Cells, it is essential to use Foetal Bovine Serm (FBS) that has had the native exosomes removed. For this application Exosome depleted FBS is perfect.
Detection & Measurement
Antibody-based Methods for Detection
Exosome-specific antibodies and antibody arrays are a simple solution for general exosome detection. It is recommended that CD63, CD9, CD81, HSP70 antibodies are used for general exosome detection, while tissue-specific exosomes can be discerned using antibodies for CETP, ANXA5-1, TSG101, EpCam, Vimentin, ALIX, FLOTILLIN-1 and the T-cell and B-cell exosomal marker CXCR4.
Antibody-based Methods for Quantitation
When using antibodies to quantify exosomes, such as with ELISAs, it is important to include a no-exosome control to ensure that antigens not associated with exosomes are not included in the measurement. For general exosome quantitation antibody-conjugated beads for FACS and ELISA kits using CD63, CD9, CD81, HSP70 antibodies can be used. In addition, there are several markers that can be used to quantify exosomes from a subset of tissues: CD31, Cd44, HLA-G and the T-cell and B-cell exosomal marker CXCR4.
Given the popularity of using Flow Cytometry for analysis and sorting of small particles and exosome characterisation (1, 2, 3), it is important to ensure the instruments are properly set up for this application. Bangs Laboratories have recently launched the NanoBead Calibration kit, which comprises highly defined microspheres with an internalised fluorescent dye to allow users to determine the capabilities of their cytometer and appropriate instruments settings. The kit is a superb size reference for small particle characterisation, and assessment of small particle handling processes.
Activity-based Methods for Quantitation
Acetyl-CoA Acetylcholinesterase (AChE) activity is known to be enriched within exosomes (4, 5). Therefore, by directly measuring AChE against a standard curve calibrated to validated exosome concentrations, the absolute number of exosomes present in a sample can be quantified. The EXOCET assay is solution-based enzymatic, colorimetric assay, read at OD405 and enables exosome quantitation in as little as twenty minutes.
Biophysical Methods
In addition to biochemical methods for exosome detection and quantitation, a number of biophysical techniques can be used. Transmission electron microscopy (TEM), scanning electron microscopy (SEM), cryo EM, and atomic force microscopy (AFM) have all been used to assess morphology and quality of exosome preparations. Optical methods, such as NanoSight’s nanoparticle tracking system, provide accurate measurements of exosome size and concentration. The NanoSight LM10 instrument is based on a conventional optical microscope and uses a laser light source to illuminate nano-scale particles within a 0.3 ml sample introduced to the viewing unit with a disposable syringe. Enhanced by a near perfect black background, particles appear individually as point-scatterers moving under Brownian motion. We are able to negotiate this service for your samples via SBI.
Discovery
RNA Profiling and Characterisation
Exosomes carry a wide variety of RNA types including microRNA (miRNA), transfer RNA (tRNA), ribosomal RNA (rRNA), messenger RNA (mRNA), and other structural and non-coding RNAs. Some of these RNAs could be used for biomarker discovery as long as those selectively packaged into exosomes can be identified. RNA profiling and characterisation by qPCR and next-generation sequencing methods are essential tools in the development of this exciting aspect of Exosome research. Kits optimised for the preparation of exosomal RNA are available.
Protein Profiling and Characterisation
Exosomes carry proteins both on their surface as well as fully enclosed within the vesicle. Like exosomal RNA, exosomal proteins play a key role in exosomal biology and are useful for biomarker discovery. SBI offer a complete solution for preparing exosomes for analysis by mass spectrometry. Their XPEP kit can be used for the generation of exosome surface peptide libraries (aka “shaving”) or for creating complete peptide libraries from isolated exosomes. Both library types are compatible with direct loading onto most Mass spec instruments.
Engineering
The most exciting development in Exosome research is that of exosome engineering. Work already being carried out suggests that by their innate purpose, exosomes can be used as the ultimate nanovehicle, delivering custom cargos into target cells. Tools already exist to allow you to package and traffic nucleic acids, and even small molecules into target cells. miRNA cane be delivered for knock down studies, small molecules for biochemical or therapeutic studies. The possibilities are endless.
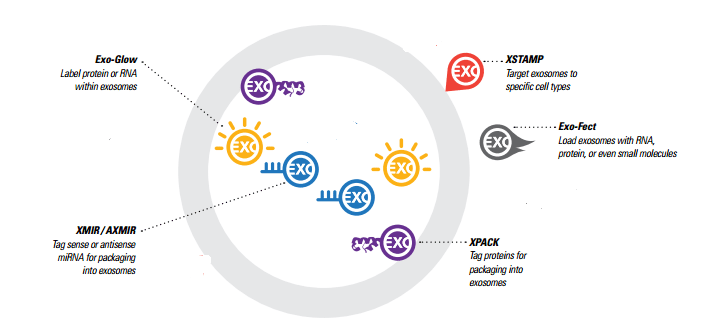
Generating Exosomes with custom cargo – Exo-Fect vs. XMIR/AXMIR
There are two different options for generating exosomes with custom RNA cargo, and the choice of which to use depends on what your experimental goals are and what materials you’re working with.
Exo-Fect is a novel nucleic acid transfer agent that enables the transfection of nucleic acids and small molecules directly into isolated exosomes in less than an hour. The Exo-Fect system can be used to deliver a wider variety of cargo to exosomes, such as plasmids, larger RNAs like mRNAs, and small molecules. The exosomes need to be either isolated or purchased first, and are then “exofected” (exosomes are “transfected”).
XMIR and AXMIR kits, allow you to simply fuse a sense (XMIR) or antisense (AXMIR) miRNA oligo to the XMIR/AXMIR RNA tag and transfect into the exosome-producing cells of your choice. For some studies, this may result in a larger supply of custom exosomes, and the XMIRXpress lentiviral system allows stable exosome packaging cell lines to be made for a constant supply of miRNA-loaded exosomes. This system is good for knocking down expression of a target in the recipient cells, or for studying other biological functions.
To summarise, the Exo-Fect system enables flexibility with cargo material and the ability to customize cargo when your exosomes are generated by difficult-to-transfect cell lines. The XMIR/AXMIR system enables generation of stable exosome packaging cell lines.
Use Exosomes as Transfection Vehicles
EV Shuttle Kits form the next generation of technology for transfection of cells that are typically resistant to many other methods (ie. Lipofectamine®). EV shuttle kits utilise exosomes for this purpose, enabling the efficient and gentle transfer of RNA and DNA into cells that previously could only be nucleofected.
Label Exosome Cargo
What happens to exosome cargo once it’s delivered to a cell? You can now find out by fluorescently labelling RNA or protein in isolated exosomes, and then delivering the exosomes to target cells. With Exo-Glow, Exo-Red for RNA and Exo-Green for proteins – the fate of exosomal RNA or proteins can be tracked once they’ve been delivered to the recipient cells.
Package Proteins inside Exosomes
XPack is the protein equivalent of XMIR/AXMIR (for miRNA), and tags proteins for incorporation into exosomes. An optimised peptide sequence targets a protein to the interior exosomal membrane, allowing the fusion protein to be packaged into exosomes for secretion. Available for immediate use are pre-made XPack-GFP and XPack-Luciferase reporters for tracking exosomes. Alternatively, you can design your own exosome-targeted protein for custom exosomes.
References
1) Chandler, W. L., Yeung, W., & Tait, J. F. (2011). A new microparticle size calibration standard for use in measuring smaller microparticles using a new flow cytometer. Journal of Thrombosis and Haemostasis, 9(6), 1216-1224. DOI: 10.1111/j.1538-7836.2011.04283.x
2) Kormelink, T. G., Arkesteijn, G. J., Nauwelaers, F. A., van den Engh, G., & Wauben, M. H. (2015). Prerequisites for the analysis and sorting of extracellular vesicle subpopulations by high‐resolution flow cytometry. Cytometry Part A. 89(2), 135-147. DOI: 10.1002/cyto.a.22644
3) Nielsen, M. H., Beck-Nielsen, H., Andersen, M. N., & Handberg, A. (2014). A flow cytometric method for characterization of circulating cell-derived microparticles in plasma. Journal of extracellular vesicles, 3. DOI: 10.3402/jev.v3.20795
4) Savina, A., Vidal, M. & Colombo, M. I. The exosome pathway in K562 cells is regulated by Rab11. J. Cell Sci. 115, 2505–2515 (2002).
5) Gupta, S. & Knowlton, A. A. HSP60 trafficking in adult cardiac myocytes: role of the exosomal pathway. Am. J. Physiol. Heart Circ. Physiol. 292, H3052–3056 (2007).