The Technology
The basic technology that underlies lateral flow immunoassays was first described in the 1960s, but the first real commercial application was Unipath’s Clearview home pregnancy test launched in 1988. Since then, this technology has been employed to develop a wide and ever-growing range of assays for clinical, veterinary, agricultural, food industry, bio-defence and environmental applications.
Strip assays are extremely versatile and are available for an enormous range of analytes from blood proteins to mycotoxins and from viral pathogens to bacterial toxins. Assays has even been developed for wine producers to assess the amount of botrytis rot in newly harvested grapes as well as for use in the clinical lab identifying cardiac markers. This shows the vast range that this technology can be applied too.
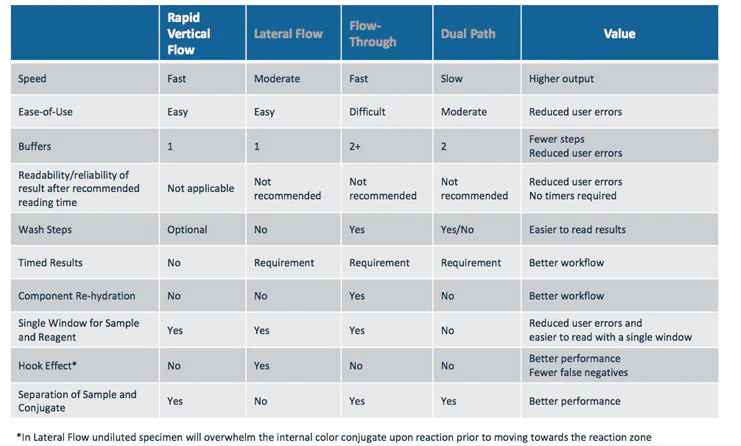
Lateral flow immunoassays are essentially immunoassays adapted to operate along a single axis to suit the test strip format. There are a number of variations of the technology that have been developed into commercial products one being Vertical Flow Technology, but they all operate using the same basic principle.
Figure 1. Advantages of lateral flow assays.
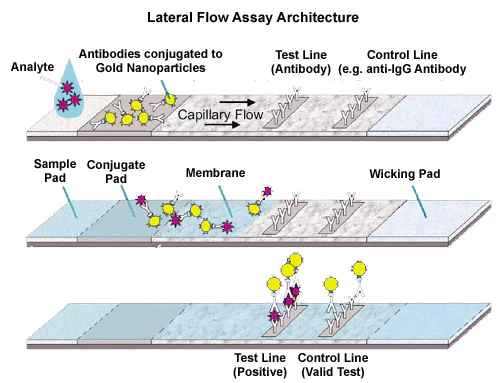
How Does a Lateral Flow Assay Work?
A typical lateral flow rapid test strip consist of the following components:
Sample pad – an adsorbent pad onto which the test sample is applied.
Conjugate or reagent pad – this contains antibodies specific to the target analyte conjugated to coloured particles (usually colloidal gold nanoparticles, or latex microspheres).
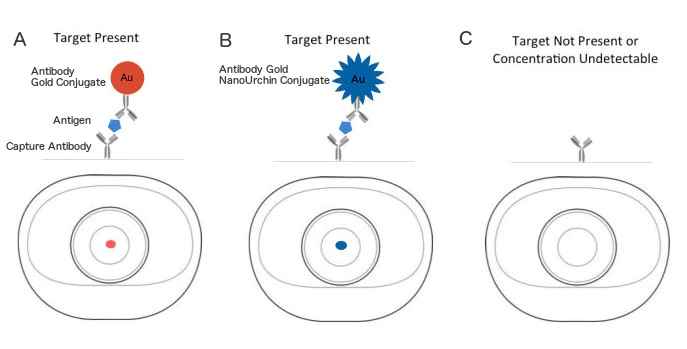
Reaction membrane – typically a nitrocellulose or cellulose acetate membrane onto which anti-target analyte antibodies are immobilized in a line that crosses the membrane to act as a capture zone or test line (a control zone will also be present, containing antibodies specific for the conjugate antibodies).
Wick or waste reservoir – a further absorbent pad designed to draw the sample across the reaction membrane by capillary action and collect it.
The components of the strip are usually fixed to an inert backing material and may be presented in a simple dipstick format or within a plastic casing with a sample port and reaction window showing the capture and control zones.
Figure 2. Principle of lateral flow assay.
Cytodiagnostics manufactures a full product line of gold nanoparticles (colloidal gold) for use in a variety of lateral flow assays. Our diverse product line of different type of nanoparticles offers you products with a narrow size distribution (CV of less than 12%), exceptional adsorption and conjugation properties and with greater than 95% spherical particles. In addition, our batch to batch variability is extremely low (+/- 2nm), which assures that you our customer will always end up with a product within the specified size range that you ordered.
The high shape uniformity of our colloidal gold will minimize the variability within your assay by e.g. allowing control over the available surface area while absorbing or covalently conjugating proteins to our gold nanoparticles. It will also ensure a more uniform flow rate across your membrane for improved reproducibility and overall results.