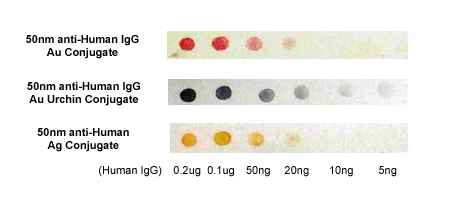
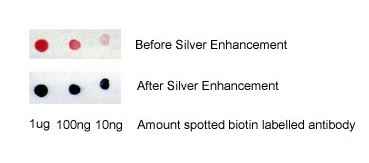
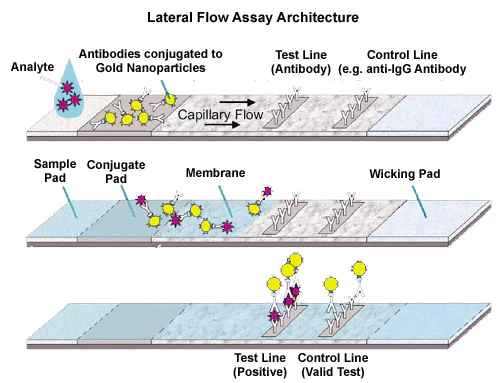
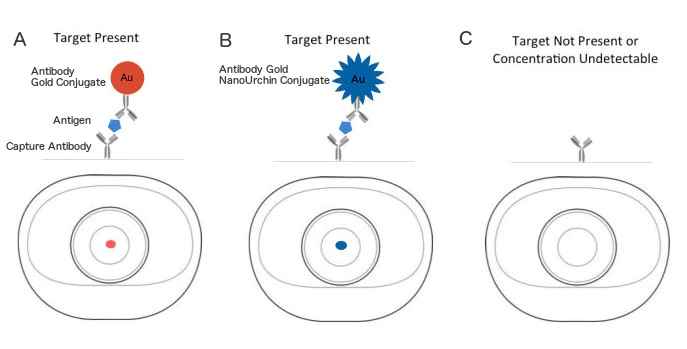
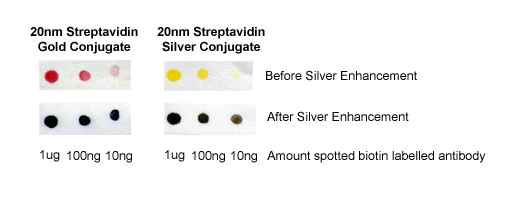
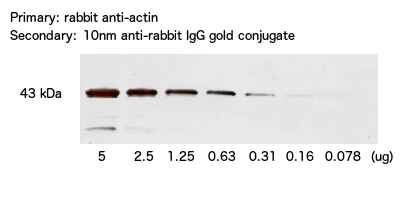
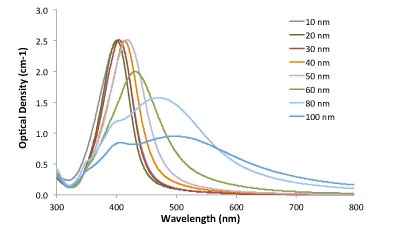
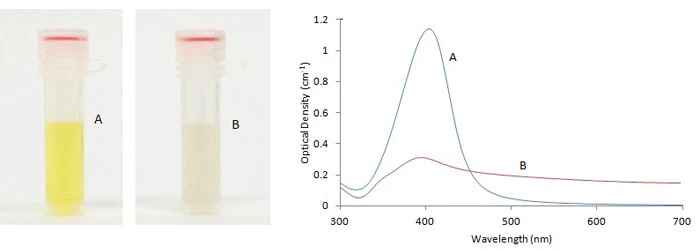
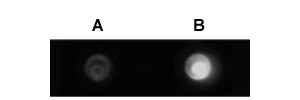
Figure 1. Immuno-dot blot assay for detection of human IgG antibodies using gold nanoparticles, silver nanoparticles and gold nanourchins (“spiky gold”) coated with bio-recognition molecules. Note how the difference in appearance (color) of the dots can be achieved using different types of noble metal protein conjugates varying in either shape or composition.
Heterogeneous Surface Design
Engineering a gold nanoparticle surface that contains PEG and bio-recognition molecules is ideal for their application. The PEG molecule prevents non-specific binding while the bio-recognition molecule provides biological specificity. Cytodiagnostics gold nanoparticle products enables researchers to design their nanoparticles in this manner, for optimum biological use.
Applications
Gold nanoparticles coated with PEG or/and bio-recognition molecules (BRM) has many applications, e.g. biosensors, cellular probes, drug delivery vehicles, or as optical contrast agents. Below, we provide some specific examples of how to use gold nanoparticle conjugates.
DNA Sensors
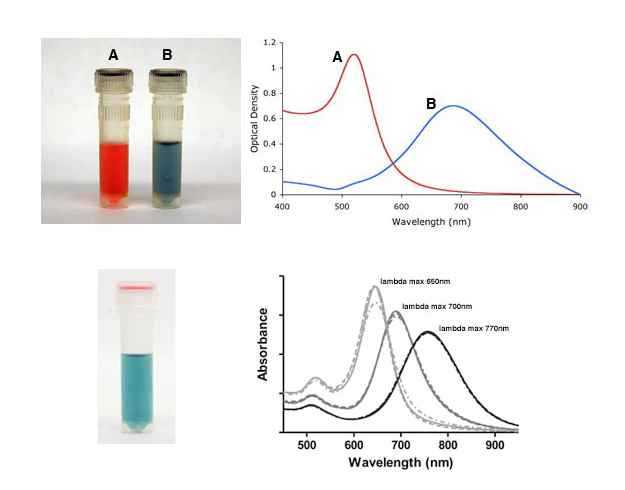
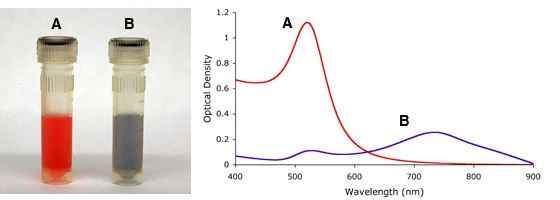
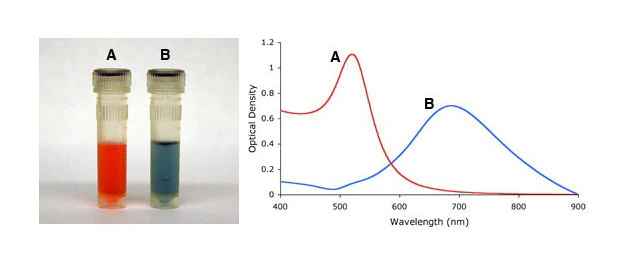
Single stranded DNA-coated onto gold nanoparticles can be used for detection of genetic material (3). In this application, single stranded oligonucleotide-coated gold nanoparticles are incubated with a DNA fragment of interest. If the fragment is complementary to the oligonucleotide sequence on the gold nanoparticles, particles are assembled together. As a result of this “aggregation” the color of the solution changes from red to blue because the surface plasmon is coupled when particles are in the aggregated state. The color change thus indicates a positive detection. Mutations can be detected by heating the sample. DNA de-hybridizes when heated and when a mutation is present in the sequence, the melting temperature is lowered. By measuring and comparing the temperature of a mutated sequence to a perfectly complementary sequence, one is able to detect whether a mutation is present or not. More complex schemes can also be designed to identify the location of a mutation within the DNA fragment analyzed.
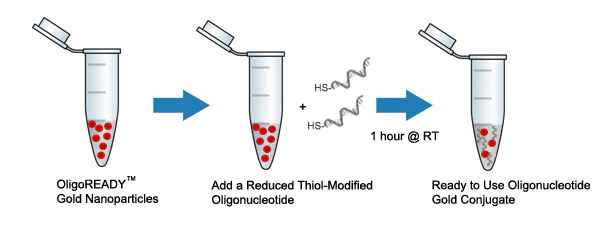
Through years of experience, Cytodiagnostics has optimized protocols for conjugation of oligos to gold nanoparticles and we are proud to offer our OligoREADY™ conjugation kits for easy conjugation of thiolated oligonucleotides to gold nanoparticles with sizes in the range of 5-100nm.
Applications in In Vivo Cancer Research
The gold nanoparticle PEG/BRM system can be designed to selectively target tumors and bind to cancerous cells. Interestingly, PEGylated gold nanoparticles can target tumors by a passive mechanism alone. An advantage of the protective PEG-layer on the gold nanoparticles is that it has been shown to reduce macrophage uptake. Macrophages are part of the reticuloendothelial system that removes foreign materials from the blood. The PEGylated layer reduces the interaction of the gold surface with blood proteins thereby minimizing non-specific macrophage uptake. This allows the nanoparticles to reside in the blood for long-term, which allows for a greater chance of tumor extravasation. If coated with a drug or imaging agent (see our Fluorescent Gold Nanoparticles), the gold nanoparticles can be used as a visualization tool as well as a delivery vehicle to the tumor. Another method of targeting tumors is by coating the gold nanoparticles with a bio-recognition molecule that recognizes receptors on tumor cells, the extracellular matrix, or blood vessels, i.e. active targeting. The advantage of using gold nanoparticles is that one can control the delivery efficiency by the size, shape, or surface chemistry (4).
Nanotoxicology
One of the key questions facing researchers is to understand how the size, shape, and surface chemistry (known as the physico-chemical properties) affect how nanoparticles distribute in the cell and body, and whether specific nanomaterial designs cause toxicity. Gold nanoparticles are ideal platforms to perform these studies on because they can be synthesized and tuned with a narrow size distribution. Further, designs with different shapes and surface chemistries can be readily achieved. This allows one to systematically evaluate their behavior in biological systems. By performing such studies, one is then able to identify the designs with the lowest toxicity, which subsequently can be selected for the final application(s).
Cellular Probes
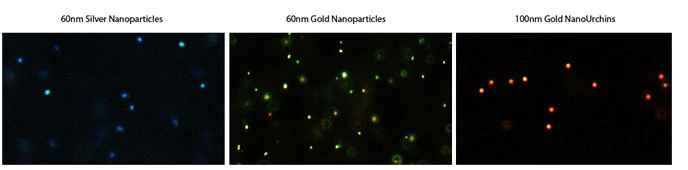
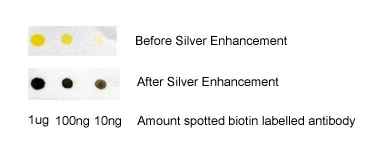
Gold nanoparticles scatter light, and when using a dark field microscope to image them, they appear as bright spots (similar to fluorescence). A unique advantage is that the dark field signal does not photobleach like fluorescence from dyes. By labeling gold nanoparticles with bio-recognition molecules such as an antibody to a cell surface receptor etc. cells can thus conveniently be targeted and imaged. In addition, low-level target detection can be improved by using silver enhancement of bound gold nanoparticles.
Summary
The surface is the interface of the nanoparticle with the environment and the ability to manipulate the surface of gold nanoparticles is essential for their use. Here, we present a product with a PEGylated surface that reduces non-specific binding to the gold surface and further functionalization with bio-recognition molecules provides an easy strategy to both target and visualize cellular targets and intracellular processes.
References
- Sperling, R. A.; Gil, P. R.;Zhang, F.; Zanella, M.; Parak, W. J.. Biological Applications of Gold Nanoparticles, Chemical Society Reviews, 2008, 37, 1896-1908.
- Walkey, C. D.; Olsen, J. B.; Guo, H.; Emili, A.; Chan, W. C. W. Nanoparticle Size and Surface Chemistry Determine Serum Protein Adsorption and Macrophage Uptake, Journal of the American Chemical Society, 2012, 134, 2139.
- Elghanian, R; Storhoff, J. J.; Mucic, R. C.; Letsinger, R. L.,; Mirkin, C. A. Selective Colorimetric Detection of Polynucleotides Based on the Distance Dependent Optical Properties of Gold Nanoparticles, Science, 1997, 277, 1078.
- Chou, L; Chan, W. C.W. Fluorescence-Tagged Gold Nanoparticles for Rapidly Characterizing the Size-Dependent Biodistribution in Tumor Models, Advanced Healthcare Materials, 2012, DOI: 10.1002/adhm.201200084.