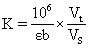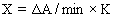1. Can the E-BC-K030 GSH assay kit detect plant tissue samples? Can it detect the ethanol extract of leaves? If it can detect, how to determine the protein concentration?
Answers:
1) It can detect plant tissue samples. It is recommended to treat the samples with PBS (0.01 M, pH 7.4) to carry out experiments, and then use BCA method to detect the total protein content in tissue homogenate, so as to calculate GSH content according to the calculation formula in the manual.
2) The ethanol extract of leaves cannot be detected.
2. E-BC-K036 NO assay kit : ① There is no significant difference between OD value of human serum and blank. ② Standard OD value is high
Answers:
1) Fresh serum is required for human serum, and the content of NO in human serum is relatively low, so little difference is normal.
2) The standard OD value is related to the standard sample amount. Because the standard sample amount of the kit is not given a fixed value, it cannot be compared with the standard OD value in the standard label given in the manual. Regarding operation: if the supernatant is not completely clarified after adding reagent 1 and reagent 2 for centrifugation, the OD value will also be high.
3. The samples in the manual are all fresh. If the samples are frozen, will it have large effect on the result? It is written in tissue sample process that it can be processed first and then placed at -80℃ for testing within one month. If it is frozen first and then processed, does it affect?
Answers:
1) It is better to use fresh samples for biochemical indicators. According to different indicators, frozen storage will have different effects on the results. For general enzyme samples, the enzyme activity after frozen storage will decrease to a certain extent compared with that of fresh samples. Some indicators, such as blood ammonia, will become higher after freezing.
2) The tissue sample should be used on the same day after homogenization. If it cannot be used on the same day, it should be placed at -80℃ for testing within one month. Freezing before treatment is better than freezing after treatment.
It should be noted that the sample should not be frozen and thawed repeatedly.
4. Does the sample need special attention during detection?
Answers:
1) For biochemical detection, the sample should be free from hemolysis, lipemia and jaundice. Tissue samples should be washed and dried before weighing.
2) After the sample is processed, it is best to detect it as soon as possible and keep it on the ice box for detection.
3) The sample needs to be clarified. If it is not clarified, the supernatant should be taken for detection after centrifugation (except for special indicators).
4) The sample value should be within the linear range of the kit. If it exceeds the linear range, it should be diluted before detection. If it is lower than the detection range, it should be detected after increasing the sample concentration.
5) For the precautions of specific sample processing, please refer to the manual.
5. How to choose when an indicator involves multiple detection methods?
Answers:
1) Detection principle.
2) Sensitivity and linear range.
3) Simplicity of operation.
4) The cost performance of the kit.
5) Whether the kit is in stock.
According to their own needs and sample value, select the appropriate detection methods based on these five items.
6. Are some kits that can be detected with spectrophotometer that can be detected with microplate reader or automatic biochemical analyzer?
Answer:
Each kit has a prescribed instrument. It is recommended to follow the instructions in the manual. Do not change the instrument at will.
7. The instructions require that experiments be carried out with a cuvette with 0.5 cm optical diameter. Can a cuvette be detected with 1 cm optical diameter?
Answer:
Theoretically, it can be detected, but its accuracy, sensitivity, detection range, intra-assay CV and inter-assay CV cannot be guaranteed.
8. What are the specifications for cuvettes?
Answer:
The cuvettes used in our kit are 1 cm optical diameter quartz cuvette (liquid volume 3 mL), 0.5 cm optical diameter quartz cuvette (liquid volume 1.5 mL), and 1 mL quartz cuvette (optical diameter 1 cm), 1 cm optical diameter glass cuvette (liquid volume 3 mL). It should be noted that glass cuvettes cannot be used in the UV range (200 nm-400 nm).
9. What are the common specifications in the kit? What does the specification mean?
Answers:
50T/48 samples: 50T means a total of 50 tests, 48 samples refer to 48 sample tubes that can be tested, and the remaining 2 tests are used for blank tubes and standard tubes/control tubes.
100T/96 samples: 100T means a total of 100 tests, 96 samples refer to 96 sample tubes that can be tested, and the remaining 4 tests are used for blank tubes and standard tubes/control tubes.
50T/24 samples: 50T means a total of 50 tests, 24 samples refer to 24 sample control tubes and 24 sample tubes, and the remaining 2 tests are used for blank tubes.
50T/36 samples: 50T means a total of 50 tests, 36 samples refer to 36 sample tubes that can be tested; the remaining 14 tests are used for standard curves.
100T/86 samples: 100T means a total of 100 tests, 86 samples refer to 86 sample tubes that can be tested; the remaining 14 tests are used for standard curves.
100T/42 samples: 100T means a total of 100 tests, 42 sample control tubes, 42 sample tubes, and the remaining 16 tests are used for standard curves.
96T:96T means 96 wells for colorimetric method detection.
10. Some homogenized medium of tissue homogenate is PBS, some is special homogenized medium. Is there any difference between different homogenized medium?
Answer:
The function of homogenized medium is to improve the extraction efficiency of indicators, reduce the loss of indicators and protect the stability of indicators during homogenate process. Try to process the sample with the homogenized medium in the instructions.
11. Why are the parallel determination results of the same sample greatly different?
Answers:
1) Sample reasons: uneven sample system, incomplete centrifugation during sample extraction, impurities and other reasons.
2) Instrument reasons: instability of the instrument results in large system error. Generally, the instrument needs to be preheated for 30 minutes before colorimetric detection.
3) Operation reasons: due to unskilled operation, sample adding and liquid adding methods, the parallel results differ greatly.
12. Why is there no difference between the sample tube and the control tube or blank tube, even lower than the control tube or blank tube?
Answers:
1) If the blank value is too high, it may be the reason that the blank tube is polluted.
2) Sample reasons: whether the sample content is very low, the sample size can be appropriately increased or the sample concentration can be increased before the experiment is carried out; whether the sample extraction is improper or not, and whether the extraction is not sufficient, it is recommended to follow the sample processing steps in the manual.
13. Why the color doesn’t appear?
Answers:
1) The sample does not contain this substance.
2) Improper pretreatment: For example, in the enzyme activity extraction process, the sample is treated with reagents that inactivate the enzyme activity.
3) Improper preservation of samples.
4) Improper storage of reagents: changes in components of the reagents, such as reagents with enzymes, which inactivate the enzyme.
14. Can addition order of the reagent be changed?
Answer:
Absolutely not. If the change is made, the reaction may not be carried out normally, leading to the failure of the experiment.
15. If the reagent is saved according to the preservation method in the manual. Can the reagent with precipitation still be used?
Answer:
Not necessarily. For details, please refer to the notes in the manual. If it is not stated in the manual, please contact customer service to answer your questions.
16. How to mix the ingredients evenly as described in the manual?
Answers:
1) The assay kit detected by spectrophotometer should be mixed evenly with vortex mixer.
2) The assay kit for the detection of the microplate reader, vortex mixer, 96-well microplate well mixer and multi-channel gun can all be used for mixing. For specific operation, please refer to the operation steps in the manual.
17. Why tissues and cells need to be detected for protein concentration? Why serum doesn’t need to be detected?
Answer:
The tissue and cells should be homogenized first, and the protein released is different with different degree of homogenate. We detect protein to correct the degree of homogenate breakage. The serum itself can be directly detected without the need for protein concentration correction.
18. If standard curve is required in the kit, can the standard curve provided in the manual of the kit be used for calculation instead of the standard curve?
Answer:
No, because the experimental environment and personnel of each laboratory is different, and the standard curve is also different. In order to ensure the accuracy of the experimental results, it is recommended to make the standard curve as required.
19. How to choose biochemical kit?
Answer:
The customer determines the index and the sample amount to be detected according to his own experimental scheme. If the same index corresponds to multiple kits, the customer should choose the appropriate products according to laboratory instruments (microplate reader, spectrophotometer and biochemical analyzer), and calculates the number of products to be purchased according to the sample amount.
20. Advantages and disadvantages of spectrophotometer, microplate reader and biochemical analyzer.
Answer:
Spectrophotometer has low price and wide wavelength range (usually full wavelength band), but the sample of the instrument is large in use and it is inconvenient to operate. The microplate reader is simple and easy to operate, but it is difficult to realize automation due to its low sensitivity, few selectable wavelengths. The biochemical analyzer has the advantages of small sample usage, simple operation, high degree of automation and accurate results, but the instrument is expensive.
21. How to store the biochemical kit?
Answer:
After receiving the kit, please read the reagent components in the manual carefully and store them according to the instructions in the manual. For reagents stored at -20℃, freeze-thaw cycles should be reduced as much as possible, while for reagents stored in dark place, make sure to avoid light during use, otherwise improper storage of reagents may result in reagent failure and incorrect detection results.
22.What types of samples can be detected by biochemical kits?
Answer:
The experimental principle of biochemical kit is based on chemical reaction and is not limited by biological species and sample types in theory. However, the extraction methods of some indexes in different samples are different. Therefore, please refer to the manual for specific details.
23. Can biochemical kits detect frozen samples?
Answer:
It is better to use fresh samples. According to different indexes, the frozen storage of samples will have different effects on the results. In general, the enzyme activity of the sample will gradually decrease or even completely deactivate with the prolonging of the freezing storage time. In some indexes, the test results of frozen samples are higher than those of fresh samples, such as blood ammonia.
The tissue homogenate sample should be detected on the same day. If it cannot be detected on the same day, it should be placed at -80℃ and detected as soon as possible. (In this case, customers are recommended to freeze the tissue directly instead of homogenizing the tissue)
Note: It is forbidden to freeze and thaw the sample repeatedly.
24. Types of commonly used anticoagulants
Answer:
1) EDTA: Its mechanism is to prevent blood coagulation by forming stable chelates with calcium ions in the aqueous phase. EDTA can affect the activity of some enzymes. Therefore, it is not recommended to use this anticoagulant when detecting the activity of target enzymes in blood samples.
2) Heparin is the best anticoagulant in blood chemical composition determination. Its anticoagulant mechanism is that it can inhibit the action between factors Ⅸa, Ⅷ and PF3 at low concentration together with anticoagulant II, and can strengthen antithrombin III to inactivate serine protease, thus preventing thrombin formation. It also inhibits the self-catalysis of thrombin and inhibits factor X. Generally, the anticoagulant dose with heparin is 10.0 ~ 12.5 IU/ml blood.
3) Citrate: Its anticoagulation mechanism is that Citric acid salt forms soluble chelate with calcium ions in blood, thus preventing blood coagulation.
4) Potassium Oxalate Monohydrate: Calcium oxalate precipitation is formed by Oxalate and calcium ions in blood, so that it has no coagulation function. Blood samples for determination of potassium and calcium content cannot be anticoagulated with Potassium Oxalate Monohydrate.
25. Preparation of serum (plasma) samples
Answers:
Both serum and plasma are blood liquid parts that do not contain visible components such as cells (including platelets). The main difference is that serum does not contain coagulation factors and platelets, while plasma contains coagulation factors. Their preparation methods are as follows:
1) Preparation of blood serum: the obtained blood cannot be anticoagulated, and is placed in a centrifuge tube or a vessel that can be centrifuged. The blood is allowed to stand still or placed in a 37℃ environment to promote coagulation. After the blood is coagulated, it is balanced and centrifuged (generally 3000rpm, centrifugation for 5-10 min). The obtained supernatant is blood serum. The supernatant can be carefully aspirated (be careful not to aspirate the cell components) and used separately.
2) Preparation of plasma: firstly, a certain proportion of anticoagulant (anticoagulant: blood = 1: 9) is added to the blood container, then the blood is added to a certain amount and mixed evenly upside down, and the supernatant obtained after centrifugation (the centrifugation conditions are the same as above) is plasma. It is better for the first user to move the supernatant to another clean container, and suck the blood plasma down gradually with a capillary suction tube against the liquid surface, so it is forbidden to suck up cellular components.
26. Cell collection method
Answer:
Suspended cells can be directly centrifuged to collect cell precipitates, and the appropriate rotating speed is selected according to cell types, generally not more than 1500 g, and the common rotating speed is 800-1000 g.
There are mainly two ways to collect adherent cells: 1) trypsin treatment, which is not recommended if the enzyme activity is detected in the sample, because trypsin will affect the detection of enzyme activity in the sample. In addition, commercial trypsin usually contains EDTA, and if the instructions state that the sample cannot contain EDTA, then cells cannot be collected by this method. 2) Cells are scraped and collected. It is recommended to use this method to collect cells.
27. Preparation of tissue or cell homogenate samples
Answers:
Tissue or cell samples are collected and homogenized after adding homogenized medium. There are 4 homogenization methods to choose from.
1) Manual homogenization: pour the sample (including homogenized medium) into the glass homogenization tube, hold the homogenization tube in the left hand, insert the lower end into the container containing ice-water mixture, insert the tamping rod vertically into the homogenization tube in the right hand, and rotate and grind up and down for dozens of times (6-8 minutes) to homogenize the tissue.
2) Mechanical homogenization: put the weighed tissue into EP tube, add homogenized medium, and grind with tissue homogenate machine at 60 Hz and 90 s under the condition of ice-water bath to make tissue homogenate. The homogenate time of skin, muscle tissue and plant tissue can be appropriately prolonged.
3) Ultrasonic crushing: ultrasonic treatment with Ultrasonic generator at an amplitude of 14 μm for 30 s, crushing cells under the condition of ice-water bath; Or use ultrasonic crusher, 200 W, 2 s/ time, 3 s gap for treatment, the total time is 5 min.
4) Repeated freezing and thawing: applicable to cell samples, i.e. resuspension of cells with hypotonic solution or double distilled water, followed by “freeze-thaw-freeze” cycle of cell suspension for about 3 times. It is worth noting that this operation method will affect the activity of some enzymes. Therefore, it is not recommended to use this method when detecting the activity of cell samples.
28. General requirements for sample detection of biochemical kits
Answers:
1) Blood samples have no hemolysis, lipemia or jaundice. Tissue samples should be rinsed clean and dried before weighing.
2) After the sample is processed, it should be placed on the ice box for detection (detect as soon as possible)
3) The sample needs to be clarified. If it is not clarified, the supernatant should be taken for detection after centrifugation (except for special indexes).
4) The sample value should be within the linear range of the kit. If it exceeds the linear range, it should be diluted before detection. If it is lower than the detection range, it should be detected by increasing the sample concentration or increasing the sample volume.
29. When detecting the same reaction substance, why different kits select different wavelengths, such as E-BC-K030 and E-BC-K051, both of which are DTNB reactions, why the detection wavelength is different? What is the difference between it and the 412nm wavelength in the market?
Answer:
The rest of the substances in the reaction system will interfere with the detection of reaction products at a certain wavelength. The concentration of each substance in the system is different, and the degree of interference will be different. Therefore, according to the concentration of the product system, we will select the most appropriate wavelength for detection.
30. Why is the detect data significantly different from literature reports or previous laboratory detection?
Answer:
1) Influence of sample itself: The concentration or enzyme activity of the same index will change greatly in different samples, different development stages or different environments of the same sample.
2) Influence of determination method: For the same index, the detection results will be greatly different due to different determination methods. When comparing with the literature, attention should be paid to the detection method, cultivation and processing conditions, calculation formula, unit, unit definition, etc.
3) Influence of operation and equipment: The sampling habits of operators, homogenization degree and external detection environment will have great influence on the detection results. The detection limit and stability of different instruments and equipment will also have certain influence.
4) When comparing the detection results of other documents, the data difference and change trend between the experimental group and the control group should be compared instead of the value of the detection results.