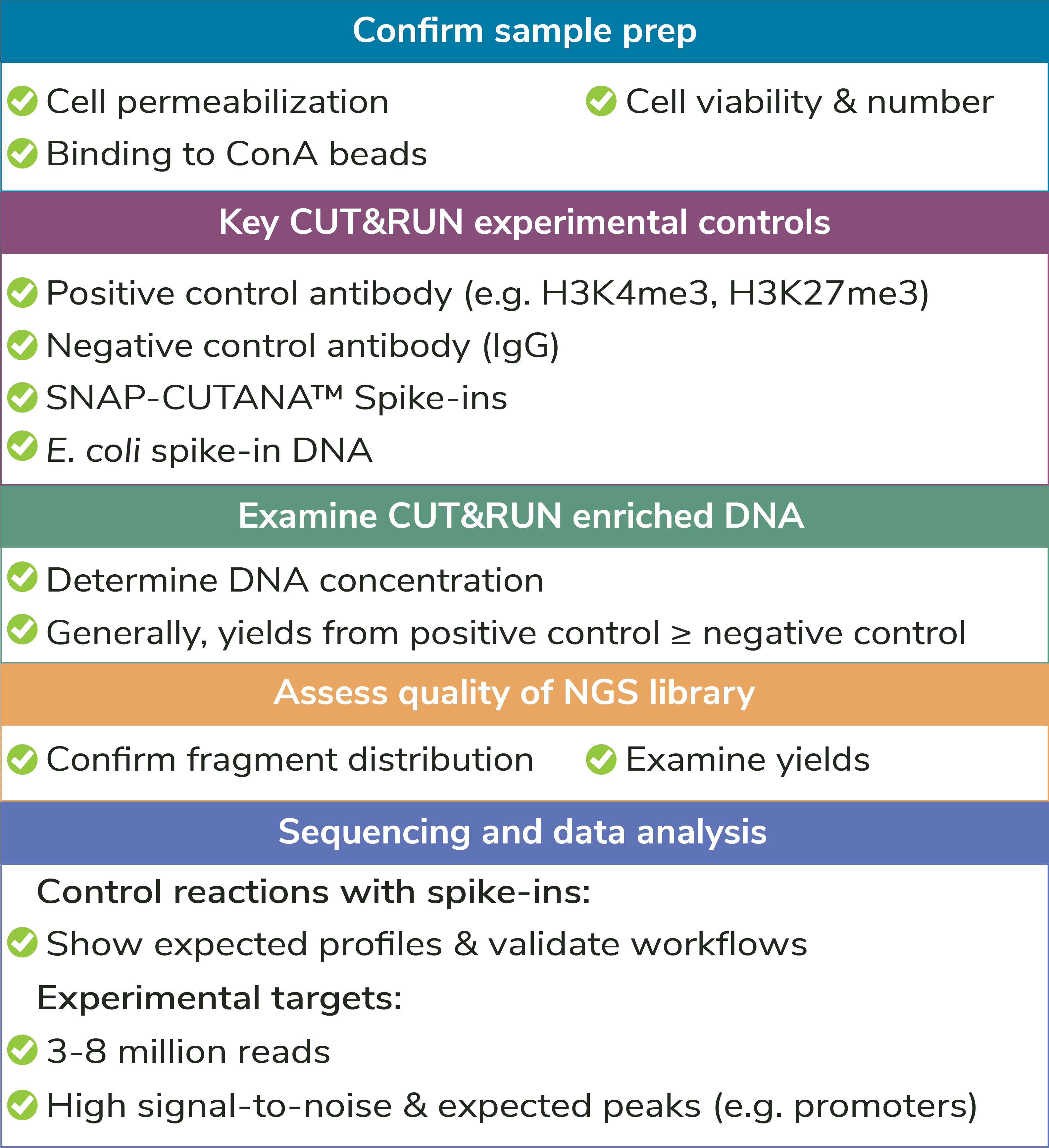
What is CUT&RUN?
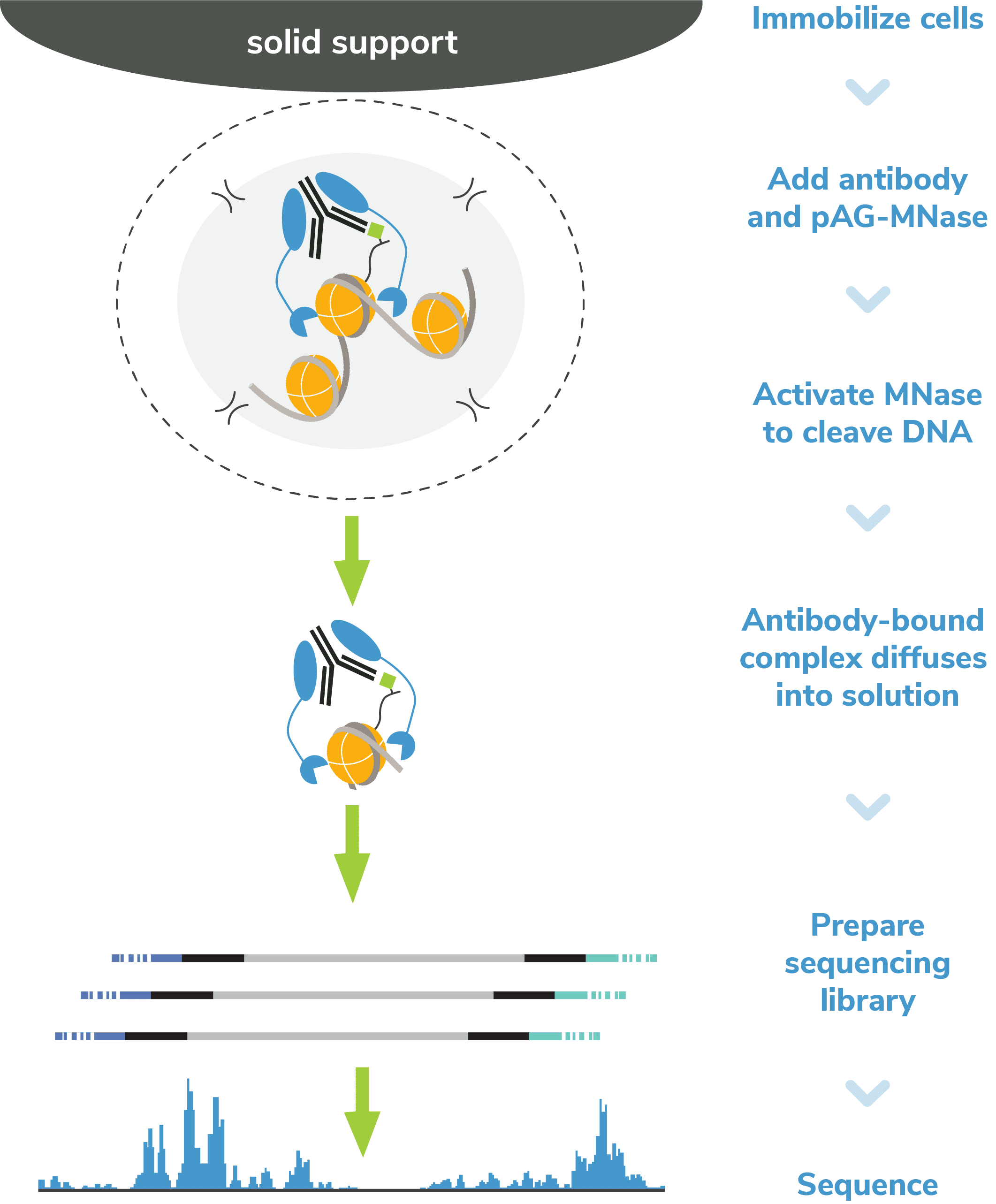
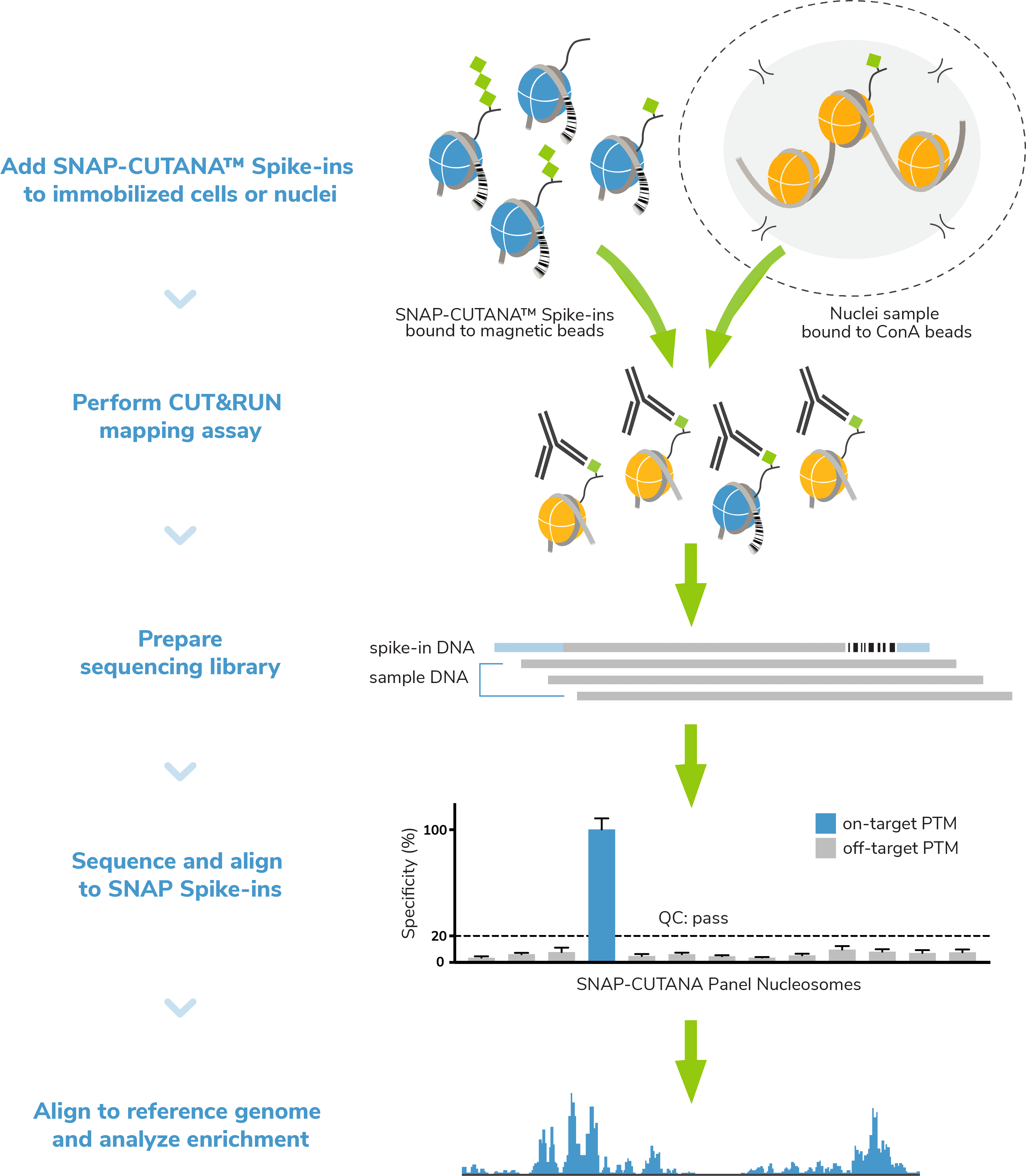
CUT&RUN, or Cleavage Under Targets and Release Using Nuclease, is a groundbreaking approach for ultra-sensitive genomic mapping of chromatin targets. CUT&RUN was developed in Dr. Steven Henikoff’s group1-3, and builds off immunotethering technology (chromatin immunocleavage, or ChIC) developed by Dr. Ulrich Laemmli4. In CUT&RUN, Protein A and Protein G are fused to micrococcal nuclease (pAG-MNase) and used to selectively cleave antibody-labeled chromatin in intact cells. The clipped fragments diffuse into solution, where they can be separated from cells, purified, and analyzed by next-generation sequencing (NGS).
CUT&RUN often draws comparisons to ChIP-seq since both assays identify the genomic location of chromatin-associated proteins (CAPs) and histone post-translational modifications (PTMs). However, CUT&RUN is fundamentally distinct from ChIP and provides a more robust approach for chromatin mapping.
What’s the difference between CUT&RUN and ChIP-seq?
- Lower cell requirements. CUTANA™ CUT&RUN assays generate high-resolution profiles using as few as 5,000 cells, and lower cell numbers have been reported in the literature5,6.
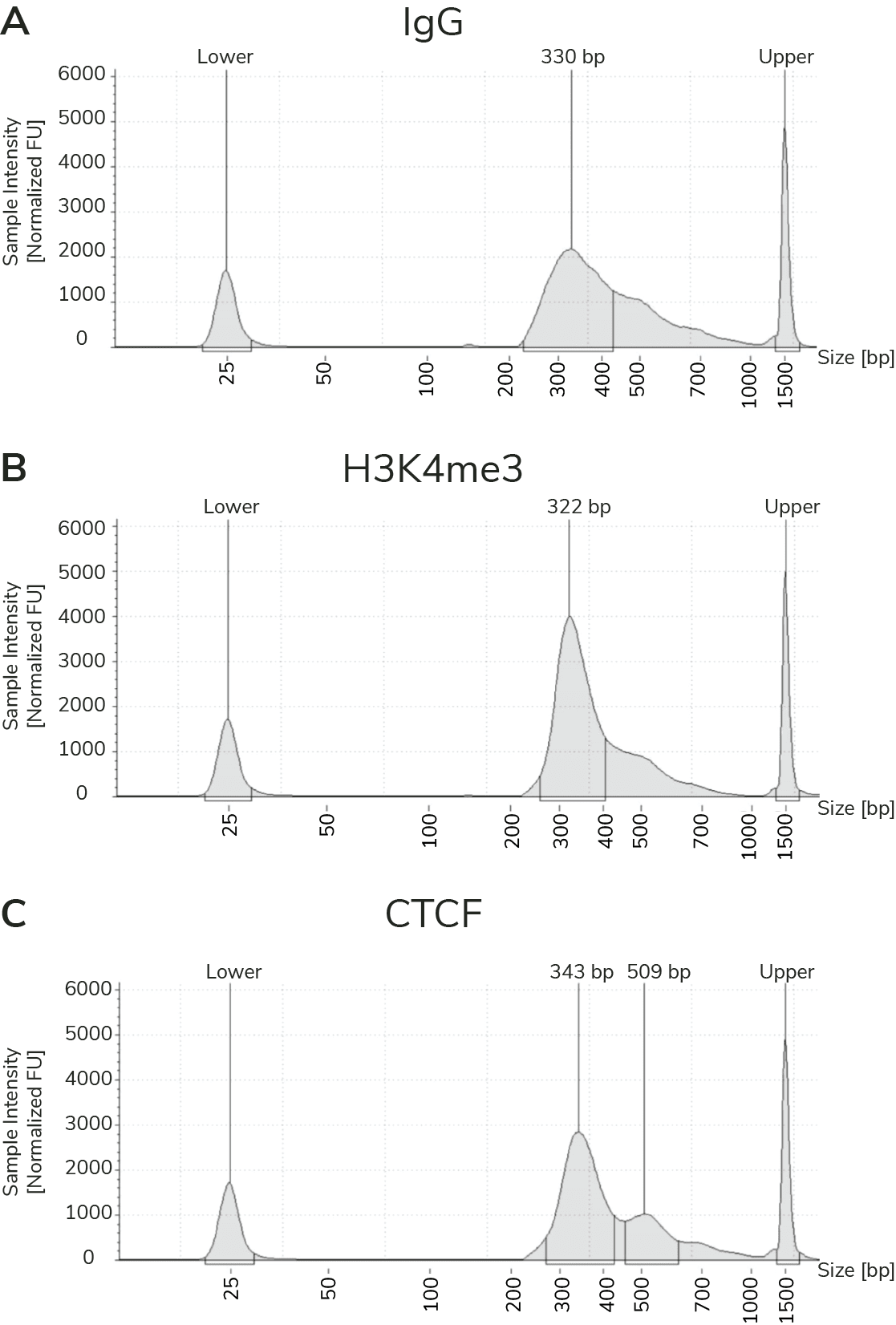
- Improved signal-to-noise. Separation of target-bound chromatin from bulk cells means lower background and robust signal – even when using fewer cells.
- Reduced cost. High signal-to-noise enables confident peak calling with ~10-fold fewer sequencing reads and less primary antibody.
- Less optimization. The entire assay is performed in intact cells (or nuclei) immobilized on a solid support. These advances eliminate the most technically challenging steps of ChIP-seq, including cell lysis, chromatin fragmentation, and immunoprecipitation (IP).
- Faster. CUT&RUN provides a user-friendly, streamlined protocol that allows scientists to go from cells to sequencing data in less than four days (vs. weeks for ChIP-seq). CUT&RUN can also be automated for greater throughput7,8.
CUT&RUN opens up entirely new avenues of research that were impractical using ChIP-seq. The low cell requirements make it feasible to analyze rare or precious samples (e.g. primary tissues, FACS-sorted cells) and to expand studies to additional targets. The increased throughput and reduced costs also support scaled epigenomics, which is key for chromatin mapping applications in clinical research and drug development. And of course, the improved signal and reduced background is a crucial advance that will drive novel applications of epigenomics datasets.