Application Examples
Browse through our application examples demonstrating, how IBA products were used in the various life science research projects conducted by both our customers and our in-house team of scientists and which results were obtained.
Browse through our application examples demonstrating, how IBA products were used in the various life science research projects conducted by both our customers and our in-house team of scientists and which results were obtained.
CD3+ T cells were isolated from buffy coats or murine splenocytes using CD3 Fab-Streps and Strep-Tactin-TACS® Agarose columns according to the affinity chromatographic Fab-TACS® approach. Purity, yield and viability were evaluated.
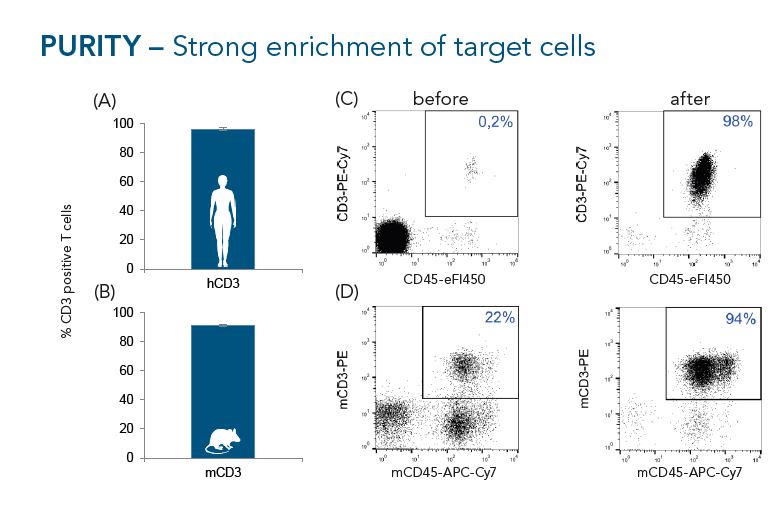
CD3+ cells were isolated from buffy coats (A, n=10) or murine splenocytes (B, n=7) using the Fab-TACS® approach for affinity chromatographic cell isolation. Data are displayed as mean ± SEM. Cells were analyzed by flow cytometry. Representative FACS plots before and after enrichment are shown in C (human) and D (mouse). Single cells were gated according to scatter properties and dead cells excluded by DAPI staining. Isolated human CD3+ T cell had an average purity of 96% and isolated murine CD3+ T cells an average purity of 91%.
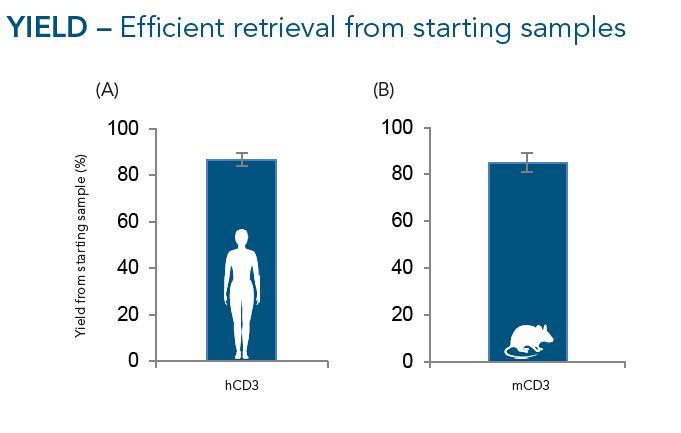
CD3+ cells were isolated from buffy coats (A, n=10) or murine splenocytes (B, n=7) using the Fab-TACS® approach for affinity chromatographic cell isolation. Data are displayed as mean ± SEM. On average, around 85% of CD3+ cells present in the starting material of both human and murine samples were retrieved after CD3-specific enrichment.
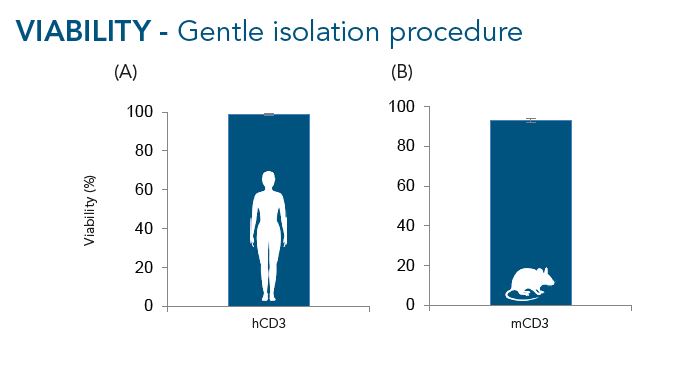
CD3+ cells were isolated from buffy coats (A, n=10) or murine splenocytes (B, n=7) using the Fab-TACS® approach for affinity chromatographic cell isolation. Data are displayed as mean ± SEM. On average, 99% of cells were viable after isolation from buffy coats and 91% after enrichment from murine splenocytes.
T cells (CD3+, CD4+ and CD8+) were isolated using Fab-Streps and Strep-Tactin® Magnetic Microbeads according to the Fab-TACS® approach for magnetic cell isolation. Purity and viabiliy were evaluated.
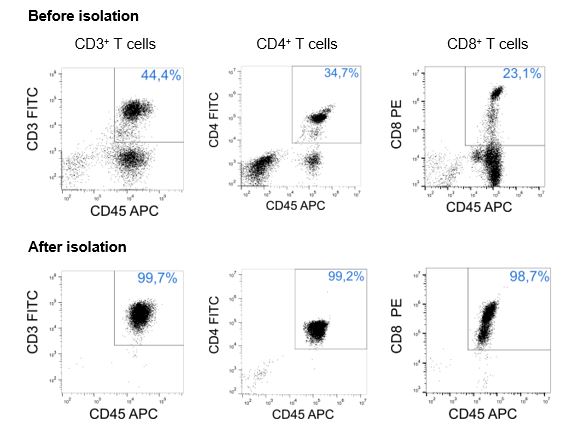
CD3+, CD4+ or CD8+ T cells were isolated from PBMCs using Fab-Streps and Strep-Tactin® Magnetic Microbeads according to the Fab-TACS® approach for magnetic cell isolation. Unlysed cells were either stained with CD3-FITC (OKT-3), CD4-FITC (SK3), or CD8-PE (HIT8a), and CD45-APC (2D1), and analyzed by flow cytometry (CytoFlex, BC). Dead cells were excluded from the analysis using DAPI staining. Doublet and debris discrimination were performed using different FSC/SSC signals. Purity was > 98% for all T cell populations after isolation.
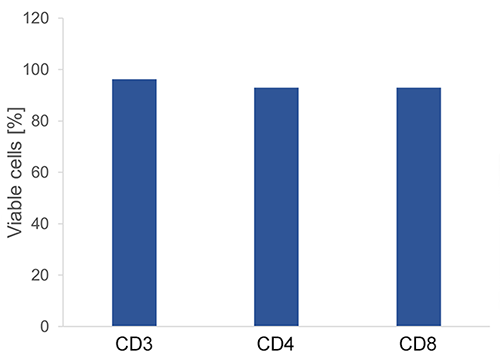
CD3+, CD4+ or CD8+ T cells were isolated from PBMCs using Fab-Streps and Strep-Tactin® Magnetic Microbeads according to the Fab-TACS® approach for magnetic cell isolation. Dead cells were determined using DAPI staining. Doublet and debris discrimination were performed using different FSC/SSC signals. > 93% of cells were viable after all isolation procedures.
The MEXi system consisting of MEXi 293E cells, pDSG-IBA expression vectors, MEXi-TM transfection medium, and MEXi-CM culture medium was applied for expression of different proteins. Subsequent purification occurred either with Strep-Tactin® Superflow® high capacity or Strep-Tactin®XT Superflow®. Yield and purity were analyzed by SDS-PAGE.
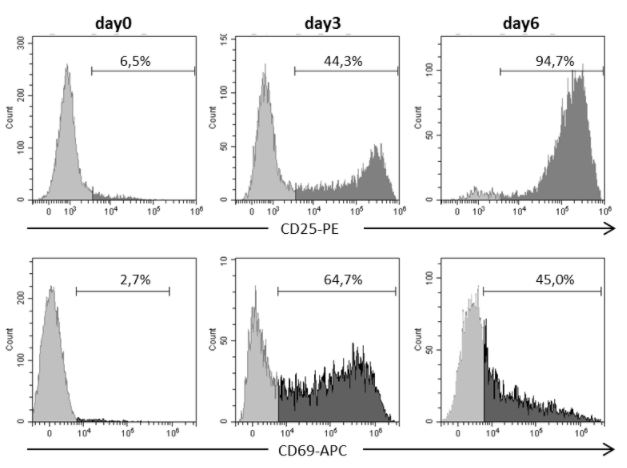
Human CD3+ T cells were isolated with CD3 Fab-Streps and Strep-Tactin® Magnetic Microbeads according to the Fab-TACS® approach for magnetic cell isolation. Activation markers CD25 or CD69 were assessed in pregated CD3+ T cells using Flow Cytometry. Dead cells were excluded from the analysis using DAPI.
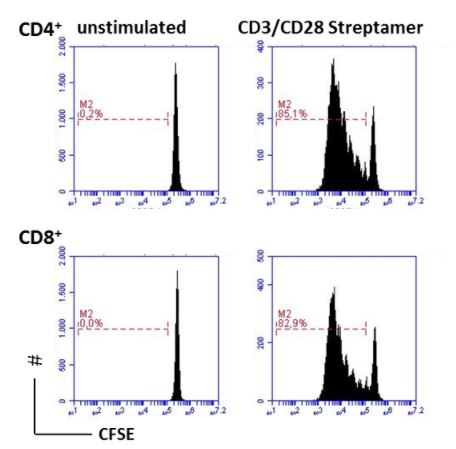
Human CD3+ T cells were isolated with CD3 Fab-Streps and Strep-Tactin® Magnetic Microbeads according to the Fab-TACS® approach for magnetic cell isolation. Cells were stained with CFSE and cultured with CD3/CD28 Streptamers in a 48 well plate for 5 days. Proliferation was measured in pregated CD4+ (upper panel) or CD8+ T cells (lower panel) using Flow Cytometry. Dead cells were excluded from the analysis using PI.
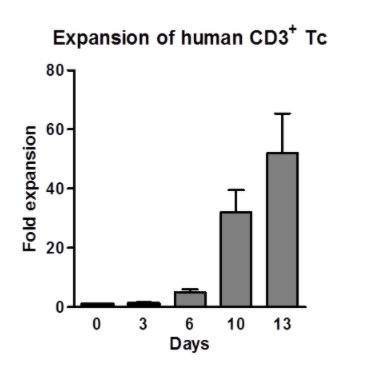
Human CD3+ T cells were isolated with CD3 Fab-Streps and Strep-Tactin® Magnetic Microbeads according to the Fab-TACS® approach for magnetic cell isolation. Cells were cultured for 13 days with CD3/CD28 Streptamers for cell expansion. Fold expansion was measured.
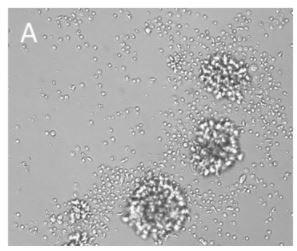
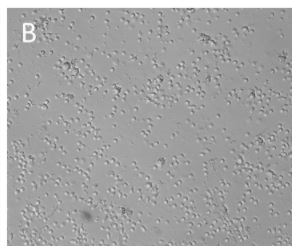
Human CD3+ T cells were isolated with CD3 Fab-Streps and Strep-Tactin® Magnetic Microbeads according to the Fab-TACS® approach for magnetic cell isolation. Cells were cultured with (A) or without (B) CD3/CD28 Streptamers for T cell expansion. Cluster formation was evaluated using microscopy.
CD3+ T cells were isolated from PBMCs using the magnetic Fab-TACS® cell isolation method. Cells were stimulated and expanded with CD3/CD28 Streptamers as indicated. T cell activation, proliferation, fold expansion and cluster formation were evaluated.

Type: metalloprotein, hydrolase
Yield : 143 mg/l.
Secreted Alkaline Phosphatase (SEAP) was fused with a C-terminal Twin-Strep-tag® and the BM40 secretion signal via cloning into pDSG-IBA102. MEXi 293E cells (1.5 x 106 cells/ml) were transfected in MEXi-TM transfection medium (17 ml) with polyethylenimine (PEI, 25 kDa). Afterwards, the cell culture was incubated for 4 hours (37 °C, 5% CO2, 125 rpm) and then diluted with MEXi-CM culture medium to reach a cell density of 0.75 x 106 cells/ml. The cells were kept at 37 °C, 5% CO2, and 125 rpm for 7 days in order to obtain high protein yields. For purification, the cells were pelleted and the supernatant, containing the SEAP protein, was harvested. Free Biotin was blocked by BioLock containing avidin. The SEAP protein was finally purified using a gravity flow Strep-Tactin® Superflow® high capacity column.
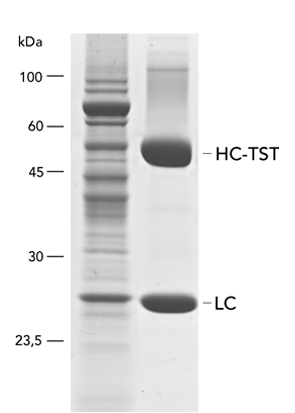
Type: antibody
Yield: 96 mg/l (represented by heavy (HC) and light chain (LC))
The monoclonal rat antibody (mAb) was cloned into the pDSG-IBA102 vector in order to fuse the heavy chain (HC) C-terminally with the Twin-Strep-tag® and the BM40 secretion signal. The transfection of MEXi 293E cells was performed in MEXi-TM transfection medium (250 ml) with polyethylenimine (PEI, 25 kDa). The cells were incubated for 4 hours (37 °C, 5% CO2, 125 rpm) and when a cell density of 3 x 106 cells/ml was reached, 250ml MEXi-CM culture medium was added. Afterwards, the culture was shifted to 32 °C and incubated until day 10.
In order to divide the cells from the supernatant, the cell suspension was centrifuged according to the MEXi manual. Free biotin was blocked by BioLock containing avidin. The supernatant was used for protein purification via a gravity flow Strep-Tactin® Superflow® high capacity column. WET FRED was used to facilitate loading of the large supernatant volume onto the column.
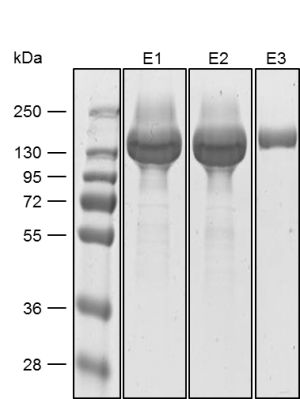
Yield: 318 mg/l
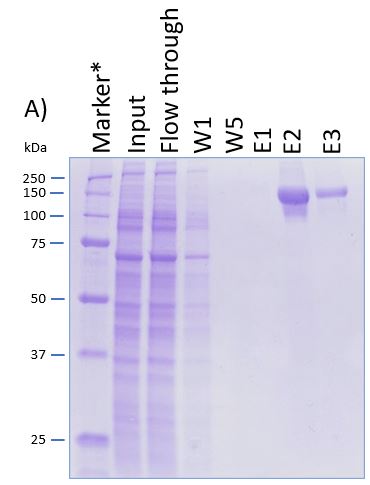
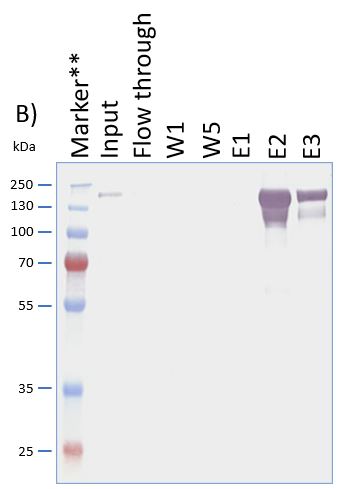
The coding sequence of the customer protein was cloned into pDSG-IBA102 leading to a recombinant protein with C-terminal Twin-Strep-tag® and BM40 secretion signal. 1050 ml of MEXi-TM was inoculated with MEXi 293E cells. Subsequently, plasmid DNA was added followed by addition of 25 kDa linear PEI. The cells were incubated for 4 hours in MEXi-TM medium at 37 °C, 5% CO2, and 125 rpm in an orbital shaking incubator. Cells were diluted to 7.5 x 105 cells/ml by addition of one volume MEXi-CM and kept at 37 °C for 7 days. Afterwards, cells were pelleted and the supernatant containing the customer protein was harvested. The customer protein was finally purified using Strep-Tactin®XT Superflow®. The figure shows the elution fractions (E1-E3). A dilution of 1:10 was prepared for E1 and E2 for SDS-PAGE analysis.
StarGate comprises vectors for expression in mammalian cells (pCSG-IBA, pESG-IBA, pDSG-IBA), yeast (pYSG-IBA), E. coli (pASG-IBA), and insect cells (pLSG-IBA). As an example, the bacterial protein azurin was cloned into different pASG-IBA vectors and expressed in E. coli. Expression results were analyzed by SDS-PAGE.
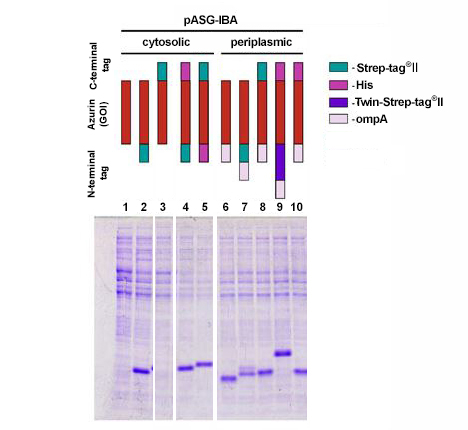
The coding sequence of azurin, a 14 kDa bacterial protein, was cloned into 10 different pASG-IBA vectors and expressed in E. coli. Comparable amounts of E. coli cells were harvested 3 hours after induction with anhydrotetracycline. Samples were lysed by boiling for 5 min at 95 °C with gel loading buffer and analyzed by SDS-PAGE. Subsequently, the gel was stained with Coomassie. Periplasmic secretion by means of ompA led in all cases to accumulation of comparable amounts of the protein of interest (lanes 6-10). This could be expected as azurin is also secreted in its authentic host P. aeruginosa. In case of cytosolic expression, however, interesting aspects became obvious, since it was not anticipated that cytosolic expression of azurin is possible at all. Expression was enabled by fusion of an N-terminal affinity tag (lanes 2, 4, and 5), while N-terminal untagged variants did not result in expression (lane 1 and 3). The examples show that initial screening for expression conditions may be worthwhile since different constructs can lead to various results depending on the target protein properties.
Exosomes were isolated from mesenchymal stem stell supernatants or human serum using Fab-TACS® for exosomes or PEGylation.
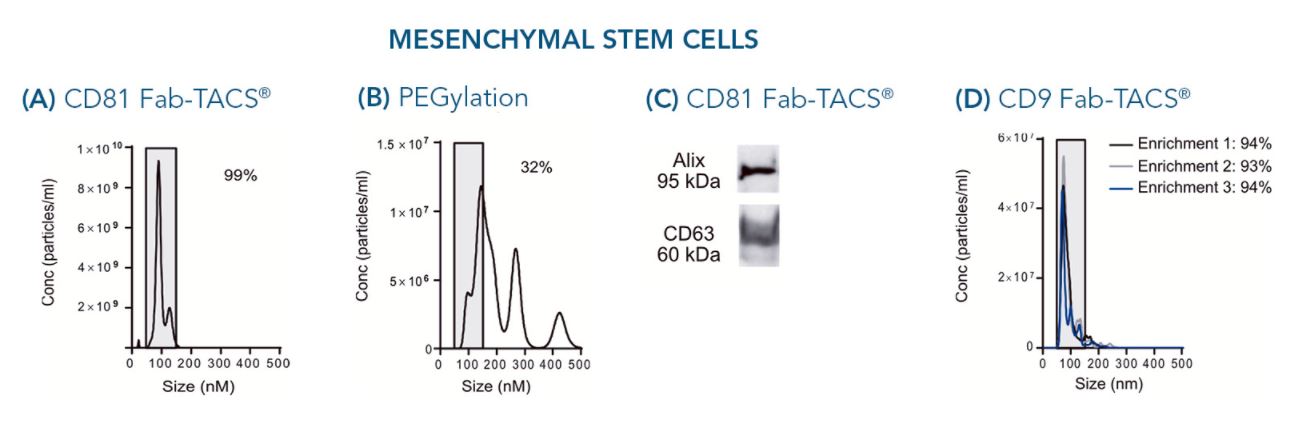
The particle size is a critical factor in evaluating the quality of isolated exosomes. 99% of particles isolated from cell culture supernatants of mesenchymal stem cells (MSCs) using Fab-TACS® for exosomes fell within the range of 30 – 150 nm (A). This indicates strong exosome enrichment. In comparison, only 32% exosome-sized particles were detected after purification with a commercially available PEGylation kit (B), implying contamination by larger extracellular vesicles.
Since other same-sized non-exosome contaminants may contribute to the pool of isolated particles, we tested for the presence of marker proteins CD63 and Alix after exosome isolation from MSC supernatant using our Fab-TACS® approach. Both proteins were clearly present within the purified particles (C), confirming their exosome phenotype.
Depending on MSC donor, cell culture supernatant compositions may vary. Purified exosomes of three independent isolations from different MSC donors were very comparable in their size ranging from 86 nm to 90 nm average diameter. All isolations yielded around 94% of particles between 30 and 150 nm in size (D).

Important exosome sources also include a variety of human bodily fluids such as blood and urine. Similar to isolations from cell culture supernatants, 96% of particles retrieved by exosome isolation from serum of humans exhibited the typical exosome size of 30 to 150 nm (E).
For protein purification of Strep-tag®II and Twin-Strep-tag® proteins with Strep-Tactin®XT IBA Lifesciences offers MagStrep “type 3” XT beads , 4Flow®, and pre-packed Spin Columns. As example, the purification of different target proteins with Strep-Tactin®XT 4Flow® and MagStrep “type 3” XT beads is shown.
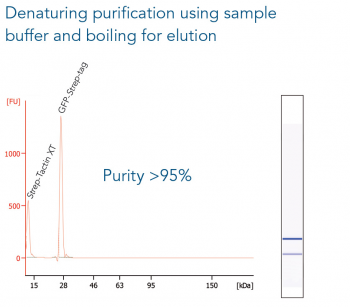
GFP C-terminally tagged with Strep-tag®II (28 kDa) was expressed in E. coli (A) and purified with MagStrep “type3” XT beads. For separation of magnetic beads from residual solution, the Magnetic Separator was used. Before elution, the sample was split and the target protein was eluted either by application of 1x Buffer BXT containing biotin or by boiling (C, 5 min at 95 °C). Due to boiling, the Beat´s agarose melts and Strep-Tactin®XT is released, leading to a further peak at 14 kDa. Protein purification results were analyzed with the Agilent Bioanalyzer 2100 system. The example shows the specific binding properties of MagStrep “type3” XT beads and the high purity that can thereby be observed.
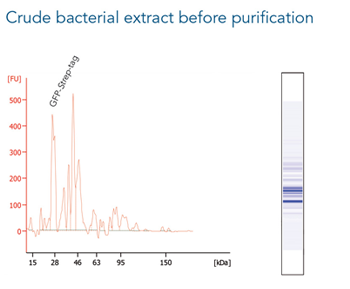
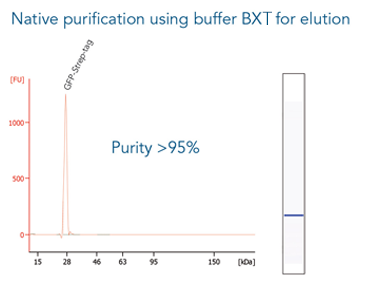
Cell culture supernatant of MEXi-293E cells (24 ml) was spiked with 240 µg of a large protein (130 kDa) C-terminally tagged with the Twin-Strep-tag®. Protein purification was carried out with a 0.2 ml Strep-Tactin®XT 4Flow® high capacity gravity flow column. After sample application the column was washed with 1x Buffer W. The Twin-Strep-tag® protein was eluted by three elution steps with 0.7, 1.5 and 0.8 CV 1x Buffer BXT. Purification results were analyzed by SDS-PAGE (A) and western blot (B). Both show a molecular weight marker (M*, Precision Plus Protein™ Unstained Protein Standards, Strep-tagged recombinant (BioRad), M** PageRuler™ Plus Prestained Protein Ladder (Thermo Fisher Scientific)), the spiked cell supernatant (S), flow through (FT), the 1st and 5th wash fraction (W1 and W5, respectively) and the elution fractions (E1-E3). Strep-Tactin® HRP was applied (1:4000) for detection via western blot.
IBA offers MacroPrep®, Superflow®, and Sepharose® coupled with Strep-Tactin® for protein purification of Strep-tag®II and Twin-Strep-tag® proteins. As example, the purification of different target proteins with Strep-Tactin® Sepharose® or Strep-Tactin® Superflow® is shown.
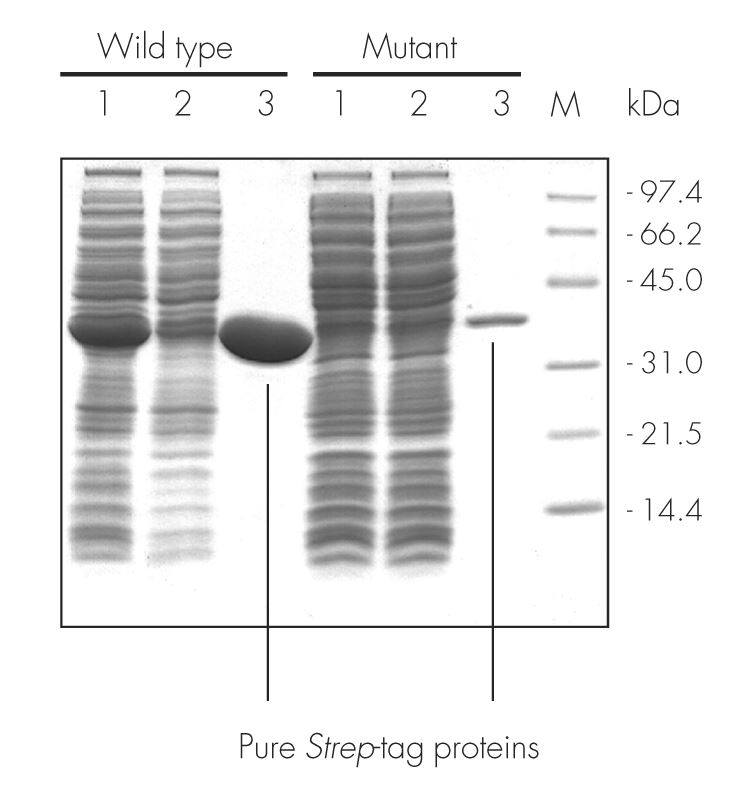
The wild type sequence and mutant variant of an enzyme were cloned into pASK-IBA3, leading to proteins localized to the cytoplasm and C-terminally tagged with the Strep-tag®II. After expression in E. coli and cell lysis, the proteins were purified with Strep-Tactin® Sepharose® gravity flow columns under physiological conditions (100 mM Tris-Cl, pH 8.0). Purification results were analyzed by SDS-PAGE. A molecular weight marker (M), cell lysate (lane 1), flow through (lane 2), and elution with 2.5 mM desthiobiotin (lane 3) are shown. For both proteins, a high purity was observed.
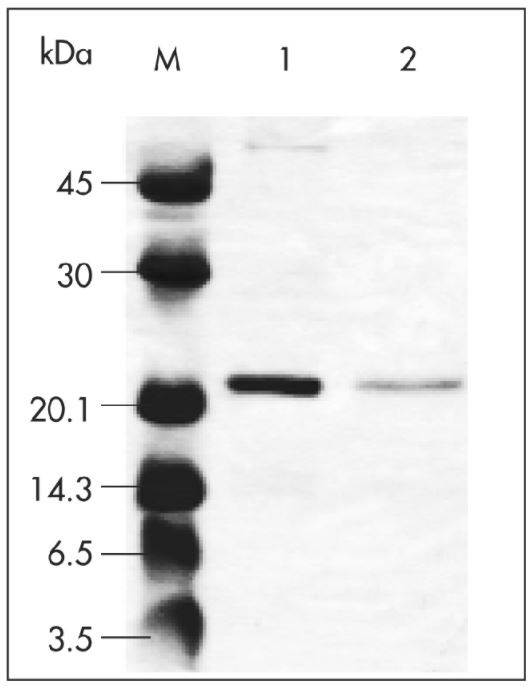
Coding sequences of virus-like particles (VLPs, 4.8 MDa) were cloned into pASK-IBA7 to obtain proteins localized to the cytoplasm and N-terminally tagged with Strep-tag®II. The plasmid was transformed into E. coli and the cell culture (1 l) was induced at OD600 of 0.6 by addition of anhydrotetracycline (AHT). Protein expression was performed at 37 °C for 3 h at 200 rpm. Afterwards, cells were harvested, resuspended with 20 ml 1x Buffer W (100 mM Tris-Cl, 150 mM NaCl, 1 mM EDTA), and sonicated. The insoluble material was pelleted, and the lysate was applied to a Strep-Tactin® Superflow® cartridge (1 ml/min flow rate). After washing, the target protein was eluted with 2.5 mM desthiobiotin. Purification results were analyzed by SDS-PAGE. Marker (M) and two different elution fractions of VLPs fused to Strep-tag®II (lane 1 and 2) are shown.
Data kindly provided by L. Stöckl and B. Brandenburg, Robert-Koch-Institute, Berlin. Brandenburg, et al. (2005): A Novel System for Efficient Gene Transfer Into Primary Human Hepatocytes Via Cell-Permeable Hepatitis B Virus–like Particle, HEPATOLOGY (42), 1300-1309.
IBA offers MacroPrep®, Superflow®, and Sepharose® coupled with Strep-Tactin® for protein purification of Strep-tag®II and Twin-Strep-tag® proteins. As example, the purification of different target proteins with Strep-Tactin® Sepharose® or Strep-Tactin® Superflow® is shown.
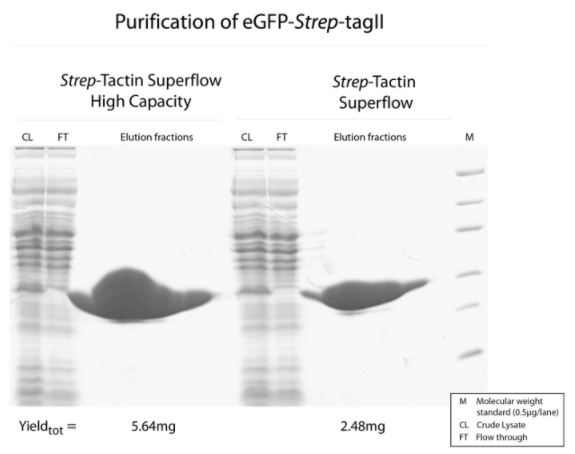
Cell lysate contaning GFP-Twin-Strep-tag was split and either purified with Strep-Tactin® Superflow® or Strep-Tactin® Superflow® high capacity.
Both purifications led to a highly pure protein, but due to the higher density of immobilized Strep-Tactin® on the high-capacity variant, a two-fold higher amount of GFP-Twin-Strep-tag can be purified with Strep-Tactin® Superflow® high capacity compared to Strep-Tactin® Superflow®.
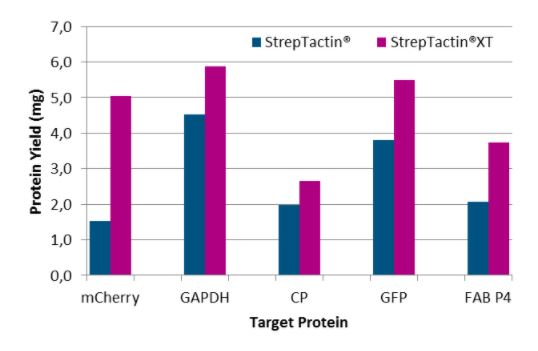
Five different proteins were purified with Strep-Tactin® Superflow® and Strep-Tactin®XT Superflow® to compare the yield with both rein types. Here, we found that Strep-Tactin®XT Superflow® allows the purification of on average a 2-fold higher protein yield than Strep-Tactin® Superflow®.
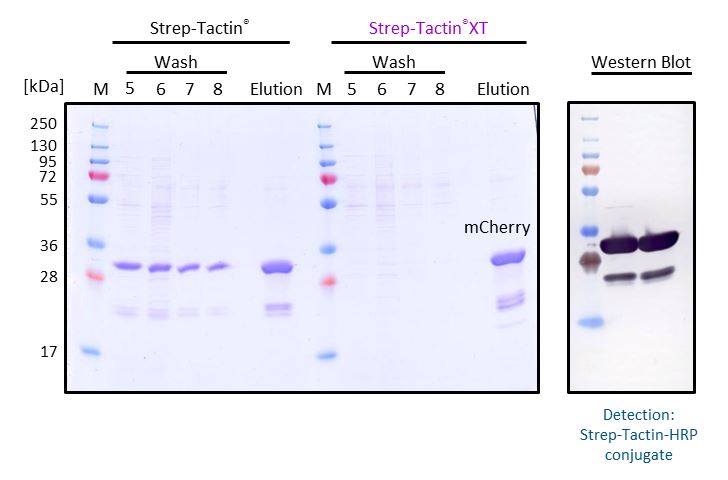
An advantage of Strep-Tactin®XT compared to Strep-Tactin® is that it allows intensive washing without loss in protein yield. To illustrate this, mCherry-Twin-Strep-tag from E. coli supernatant was purified with a gravity flow column either containing 1 ml Strep-Tactin® Superflow® or Strep-Tactin®XT Superflow®. During purification, the columns were washed with 8 column volumes of 1x Buffer W to reach a constant absorption ratio at A260/280 nm. Then, the target protein was eluted with 50 mM biotin. Samples of the last wash fractions (wash 5-8) and the elution fraction were analyzed by SDS-PAGE. In comparison to Strep-Tactin® Superflow®, Strep-Tactin®XT Superflow allows intensive washing without loss of protein yield. The high affinity (pM range) between Strep-Tactin®XT and Twin-Strep-tag® leads to a tighter binding and prevents a premature elution of the target protein during the wash steps. The elution fractions were further analyzed by Western Blot and detected with Strep-Tactin®HRP, showing that the further bands are alternative variants of mCherry-Twin-Strep-tag.
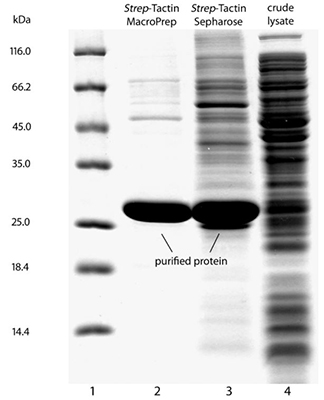
For some recombinant proteins, for example from plants, purification results of Strep-Tactin® MarcoPrep® can differ from those of Strep-Tactin® Sepharose®, due to different resin properties. The SDS-PAGE gel shows the purification of a recombinant protein which exhibits exceptionally high non-specific protein binding. In this case, contaminants were almost completely removed by application of Strep-Tactin® MacroPrep®, whereas the contaminants co-eluted with the recombinant protein after the same purification protocol using Strep-Tactin® Sepharose®. Please note, normally behaving recombinant proteins can be purified in a single step to homogeneity using Strep-Tactin® Sepharose®, however, the example shows that it may be reasonable to change the resin if non-specific protein binding occurs.
Protein A allows the the tag-free purification of IgG class antibodies. Dynamic binding capacity and stability were evaluated.
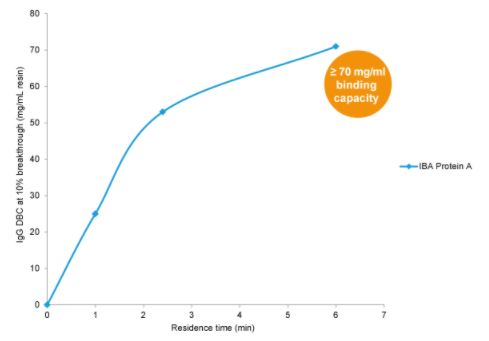
Dynamic binding capacity (DBC 10%) of IBA Protein A determined with a monoclonal antibody.
Per milliliter Protein A resin more than 70 mg of the monoclonal antibody can be captured and purified.
.

Dynamic binding capacity of Protein A remains stable after treatment with 0.5 M NaOH over~100 cycles (1 cycle = 15 min).
Strep-Tactin®XT binds with high affinity Twin-Strep-tag® proteins, making it perfectly suitable for SPR analysis. The examples show the coupling of Strep-Tactin®XT on different SPR chips and possible regeneration conditions for re-use of Strep-Tactin®XT-coated chips.
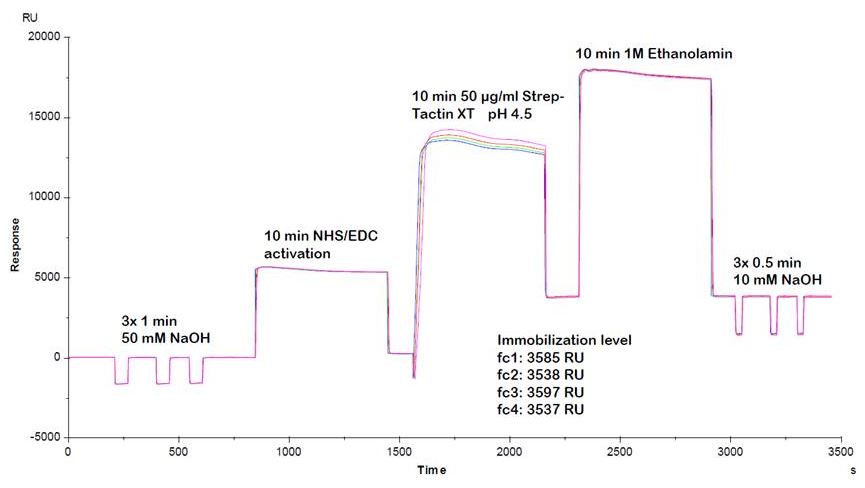
We recommend to use the following conditions for optimal Strep-Tactin®XT coupling:
20-50 µg/ml Strep-Tactin®XT
max. 20 mM acetate, pH 4.5
Using these conditions for Strep-Tactin®XT coupling, efficient and reproducible coupling results can be obtained.
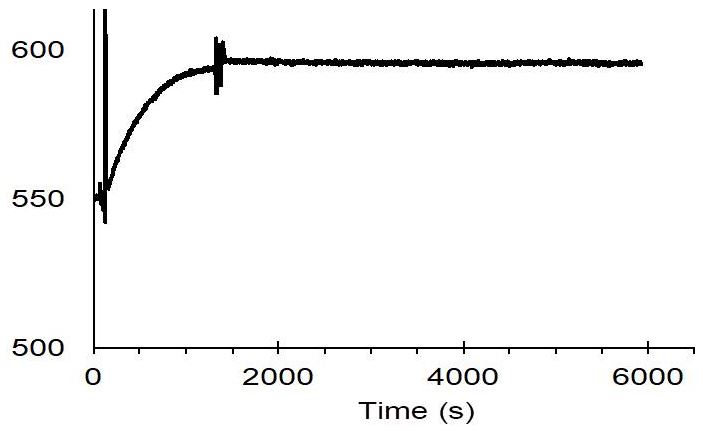
The membrane protein CB2 was fused with Twin-Strep-tag® and then immobilized on a CM4 sensor chip coated with Strep-Tactin®XT.
Used conditions:
50 mM Tris pH 7.5, 100 mM NaCl
10 % glycerol, 0.1 % DDM, 0.5 % CHAPS, 0.1 % CHS
Data was published in A. Yeliseev et al., Protein Expression and Purification 131 (2017) 109-118
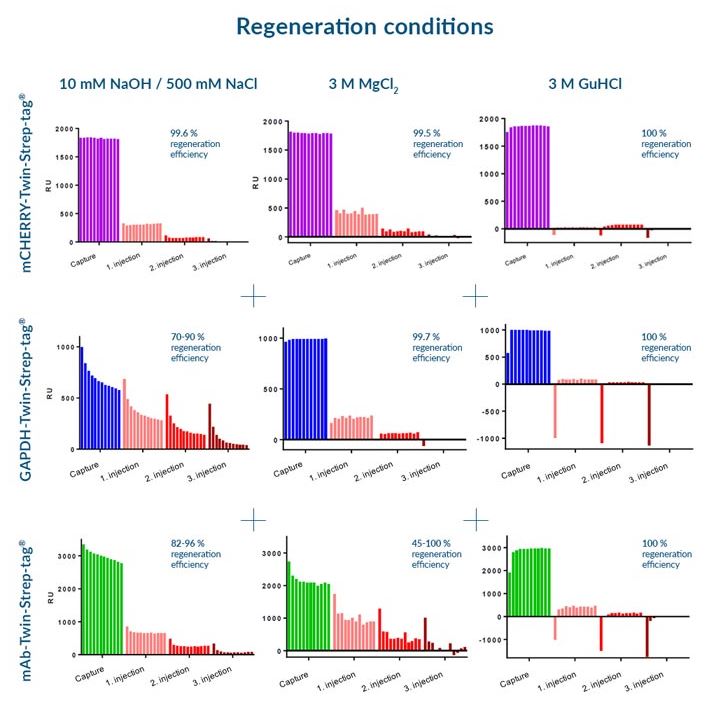
Three different regeneration conditions were tested on Strep-Tactin®XT CM5 sensorchips:
10 mM NaOH / 500 mM NaCl
3 M MgCl2
3 M GuHCl
for three different Twin-Strep-tag® fusion proteins:
mCherry-Twin-Strep-tag
GAPDH-Twin-Strep-tag
mAb-Twin-Strep-tag
We recommend the application of 3 M GuHCl for optimal regeneration conditions, since this buffer led to the best regeneration results for all test proteins. However, depending on the target protein, other buffers are applicable as well.
Due to the nearly irreversible binding of Strep-tag®II proteins, StrepMAB-Immo can be used for SPR analysis. The example shows the stable capture of a Strep-tag®II fusion protein on a SPR chip coated with StrepMAB-Immo.
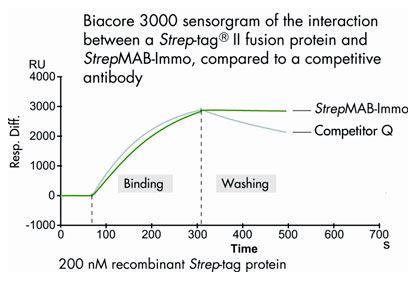
Chips for SPR were coated either with StrepMAB-Immo or with a competitive antibody (Competitor Q). Subsequently, a Strep-tag®II fusion protein was captured, and the binding stability was determined using a Biacore 3000.
During the washing phase, the recombinant Strep-tag® protein of interest remains tightly bound to StrepMAB-Immo, while a significant amount of Strep-tag® protein is washed off using the competitive antibody.
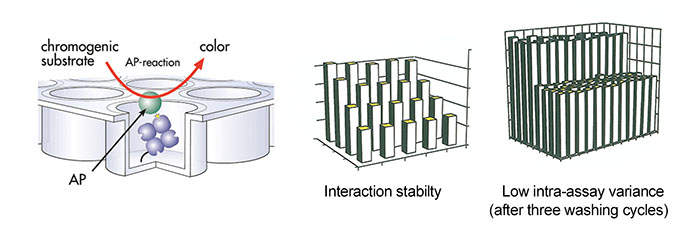
Strep-tag®II fusion proteins were immobilized on Strep-Tactin® microplates. Subsequently, the activity or abundance of the Strep-tag®II fusion proteins was determined by enzymatic reaction.
Strep-Tactin® coated microplate was incubated with different amounts of recombinant E. coli alkaline phosphatase (AP) tagged with the Strep-tag®II. After several washing steps, activity of bound fusion protein was determined by colorimetric reaction.
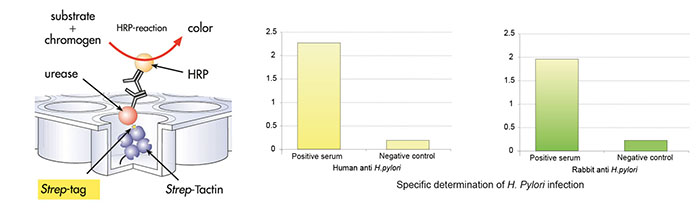
Strep-Tactin® coated microplate was incubated with recombinant H. pylori urease tagged with Strep-tag® II, followed by three washing cycles. Afterwards, the microplate was incubated with human sera followed by three washing cycles. Third incubation occurred with rabbit anti-human lgG conjugated to horseradish peroxidase (HRP), followed by 3 washing cycles. Amount of bound antibodies from human sera was determined by colorimetric reaction of HRP.
Strep-Tactin® conjugates can be used for several assay applications. As example, the application of Strep-Tactin®HRP and Strep-Tactin®AP for Western Blot as well as Dot Blot is shown.
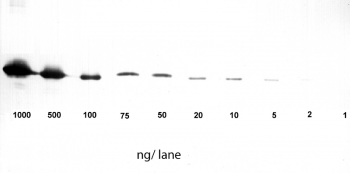
Application: Western Blot
1-1000 ng/lane of GFP C-terminally tagged with Strep-tag®II (28 kDa) were separated by SDS-PAGE and blotted onto a membrane. Detection occurred with Strep-Tactin® HRP.
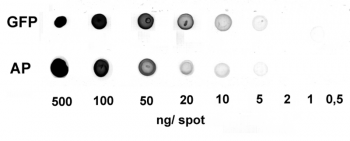
Application: Dot Blot
0.5-500 ng/spot of GFP-Strep-tagII (28 kDa) or alkaline phosphatase C-terminally tagged with Strep-tag®II (monomer 48.5 kDa) were spotted onto a membrane. Detection occurred with Strep-Tactin® HRP.
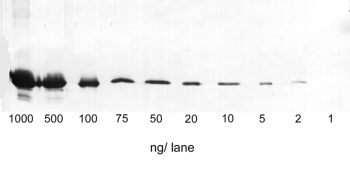
Application: Western Blot
1-1000 ng/lane of GFP C-terminally tagged with Strep-tag®II (28 kDa) were separated by SDS-PAGE and blotted onto a membrane. Detection occurred with Strep-Tactin® AP.
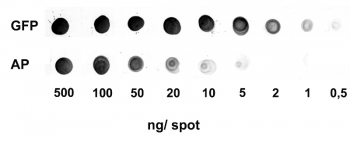
Application: Dot Blot
0.5-500 ng/spot of GFP-Strep-tagII (28 kDa) or alkaline phosphatase C-terminally tagged with Strep-tag®II (monomer 48.5 kDa) were spotted onto a membrane. Detection occurred with Strep-Tactin® AP.
StrepMAB-Classic and StrepMAB-Immo can be used for detection of Strep-tag®II or Twin-Strep-tag® proteins on cell surfaces, within the cell, in cell lysates, or in eluates after protein purification. As example, the application for flow cytometry and confocal microscopy is shown
Application: Flow cytometry
Dilution: 1:500
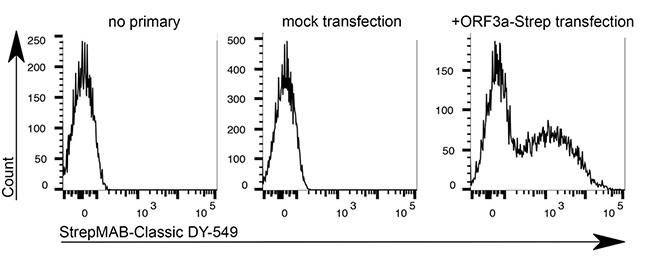
HeLa cells were transiently transfected with the coding sequence of SARS-CoV-2 ORF3a-Twin-Strep-tag. After 48 hours transfection, cells were detached, gently trypsinised and stained with StrepMAB-Classic DY-549 (1:500, 0.5% BSA + 1 mM EDTA, 30 min on ice). Intracellular SARS-CoV-2 ORF3a-Twin-Strep-tag levels were measured by flow cytometry (4 laser Cytoflex S, Beckman Coulter)
Source: Data kindly provided from James Edgar, Department of Pathology at the University of Cambridge.
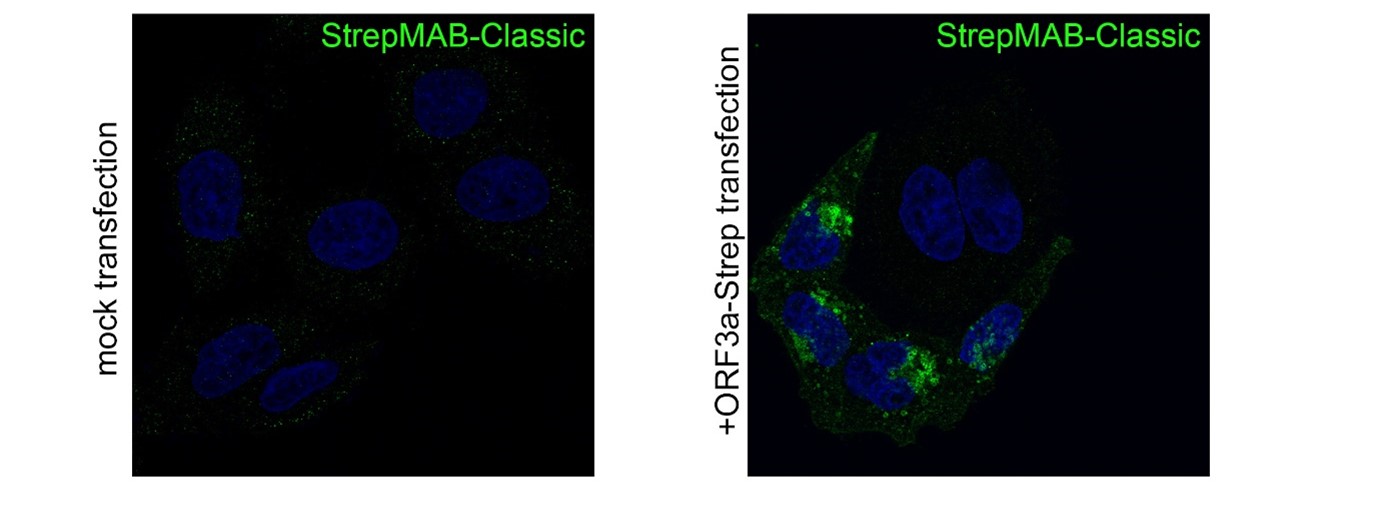
Application: Confocal microscopy (Immunofluorescence)
Dilution: 1:500
HeLa cells were grown on glass coverslips, fixed (4% PFA/PBS), quenched (15 mM glycine/PBS), permeabilized (0.1% saponin/PBS), blocked (1% BSA, 0.01% saponin in PBS), and stained with StrepMAB-Classic (1:500) to confirm the expression of SARS-CoV-2 ORF3a-Twin-Strep-tag. Anti-mouse 488 was used as secondary antibody. Nuclei were stained with DAPI and the imaging occurred with a LSM700 confocal microscope (63×/1.4 NA oil immersion objective; ZEISS).
Source: Data kindly provided from James Edgar, Department of Pathology at the University of Cambridge.
Different antigen-specific T cells were stained with MHC I-Streps and fluorescent Strep-Tactin® conjugates. All experiments were performed in close cooperation with D. Busch, TU Munich.
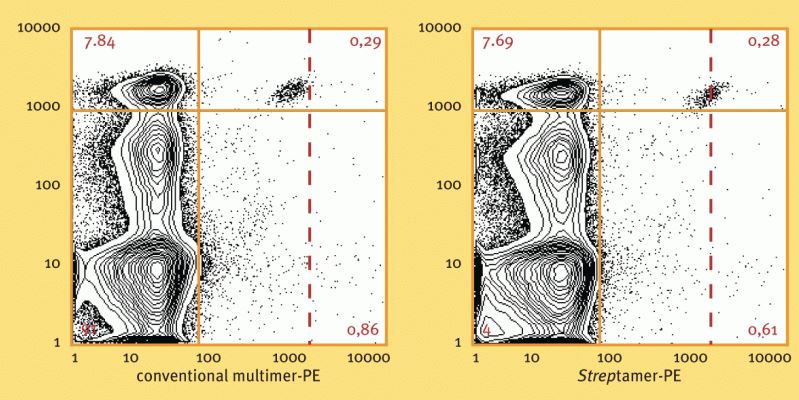
Ex vivo staining of CMV-specific CD8+ T cells with HLA-A*0201 CMV pp65495-503 Streptamer® (right diagram) or conventional multimers (left diagram). Staining was performed from a CMV positive individual at 4°C. Staining with MHC I Streptamer® resulted in improved staining intensity.
Used reagents:
6-7001-001 MHC I-Strep CMV, pp65495-503
6-5000-001 Strep-Tactin® PE
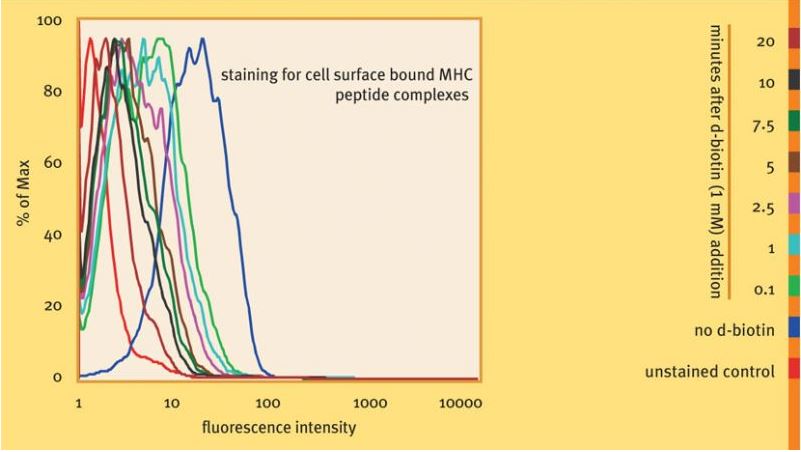
Antigen-specific cells were visualized with Strep-Tactin® PE coupled to MHC I-Strep H2-Kd specific for LLO91-99 . After addition of 1 mM d-biotin, subsequent dissociation of monomerized MHC molecules was monitored at different time points as indicated. 20 minutes after biotin addition, staining reagents were largely removed from the cells. Staining and dissociation were performed at 4 °C.
Used reagents:
6-7014-001 MHC I-Strep H2-Kd, LLO91-99
6-5000-001 Strep-Tactin® PE
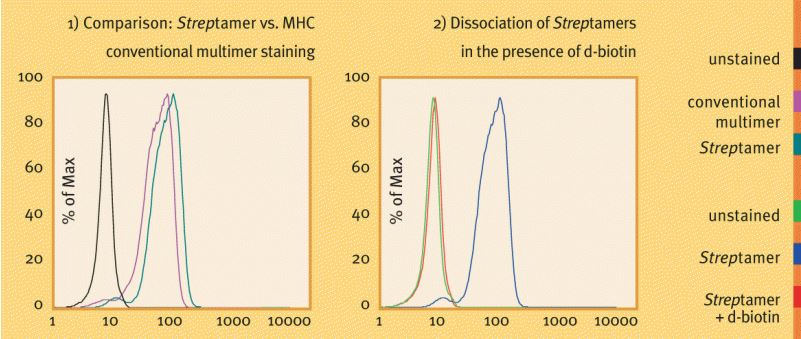
Staining of Her2/neu-specifc CD8+ T cells (kindly provided by H. Bernhard, TU Munich) with HLA-A*0201/Her2/neu369-377 Streptamers® as compared to conventional multimers (left diagram). Complete dissociation of Streptamer® staining reagents from the cells is shown after a brief incubation (30 min) in the presence of 1 mM d-biotin (right diagram). Antigen-specific T cells were visualized with Strep-Tactin® PE coupled to MHC I-Strep HLA-A*0201 specific for Her2/neu369-377. Staining and dissociation were performed at 4 °C.
Used reagents:
6-7008-001 MHC I-Strep HLA-A*0201, Her2/neu369-377
6-5000-001 Strep-Tactin® PE