Strep-Tactin® 4Flow® high capacity replaces:
- Strep-Tactin® Sepharose®
- Strep-Tactin® Superflow®
- Strep-Tactin® Superflow® high capacity
providing identical performance and unifying the resins.
Strep-Tactin® 4Flow® high capacity replaces:
providing identical performance and unifying the resins.
Strep-Tactin® 4Flow® high capacity consists of a low concentrated (4%) and pressure stable agarose coupled with high Strep-Tactin® density. These specifications lead to
with the usual high target purities. In addition, this new resin is characterized by a superior stability enabling at least 50 regeneration cycles.
The underlying matrix of many protein purification resins are porous agarose beads. The characteristics of these agarose beads determine for which proteins and for which applications the resin is most suitable. IBA’s Sepharose® resin consists of a 4% cross-linked agarose and is therefore suitable not only for small, but also for large proteins. However, a disadvantage of Sepharose® is that it is not pressure stable and consequently not suitable for automated applications such as FPLC. In contrast, IBA’s Superflow® products work well for FPLC. The disadvantage of Superflow® is that it consists of a 6% cross-linked agarose. This means that the pores are smaller and not accessible for larger proteins, resulting into low yields.
4Flow® combines the advantageous characteristics of Sepharose® and Superflow®. It is a 4% cross-linked agarose, and it is pressure stable, making it a universal matrix for small and large proteins as well as for different applications such as FPLC.
| Matrix | Strep-Tactin® Superflow® | Strep-Tactin® Sepharose® | Strep-Tactin® 4Flow® |
| Bead structure | 6% agarose, crosslinked | 4% agarose, crosslinked | 4% agarose, highly crosslinked |
| Bead size | 60-160 μm, spherical | 45-165 μm | 50-150 μm, spherical |
| Exclusion limit | 6 x 106 Da | ~3 x 107 Da | 3 x 107 Da |
| Recommended flow rate | 1 ml FPLC column: 0,5-1 ml/min 5 ml FPLC column: 1-3 ml/min | gravity flow | 1 ml FPLC column: 0,5-1 ml/min 5 ml FPLC column: 1-3 ml/min |
| pH range for protein binding | 7-8 | 7-8 | 7-8 |
| Max pressure | 9.6 bar | gravity flow | 3.5 bar |
| Eluent | desthiobiotin | desthiobiotin | desthiobiotin |
| Regeneration buffer | Buffer R | Buffer R | 100 mM NaOH |
The increased density of Strep-Tactin® molecules immobilized on Strep-Tactin® 4Flow® high capacity translates to an overall high binding capacity compared to Strep-Tactin® Sepharose® and Strep-Tactin® Superflow® high capacity. While Strep-Tactin® Superflow® may exhibit a low binding capacity for large proteins due to its 6% agarose matrix, Strep-Tactin® 4Flow® high capacity maintains a consistent high binding capacity for all proteins independent from their size.
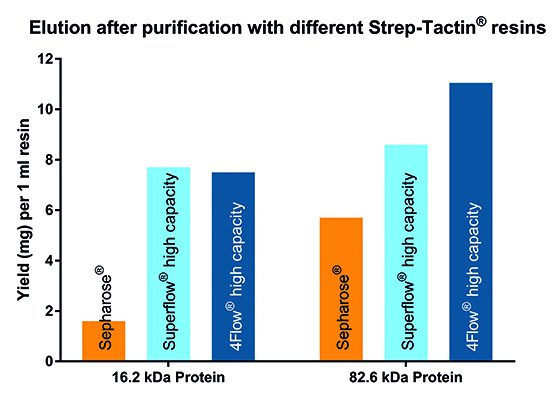
Fig.1 : Target protein yield after purification with Strep-Tactin® resins
The increased density of Strep-Tactin® molecules immobilized on Strep-Tactin® 4Flow® high capacity translates to an overall high binding capacity compared to Strep-Tactin® Sepharose® and Strep-Tactin® Superflow® high capacity. While Strep-Tactin® Superflow® may exhibit a decline in binding capacity for large proteins due to its 6% agarose matrix, Strep-Tactin® 4Flow® high capacity maintains a consistent high binding capacity for all proteins independent from their size.
Due to the specific binding of Strep-Tactin® to Strep-tag®II or Twin-Strep-tag®, a high target purity is achieved. Since the Strep-Tactin® molecules that are coupled to the new 4Flow® resin are the same as those used for Sepharose® and Superflow®, the outstanding purity of any purified protein remains identical.
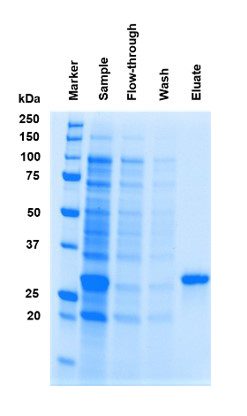
Fig. 2: High target protein purity with Strep-Tactin® 4Flow® high capacity. To evaluate the purity of the new Strep-Tactin® 4Flow® high capacity resin, a GFP protein fused to a Twin-Strep-tag® was mixed with E. coli lysate and subsequently purified using FPLC columns. A purity of 98% was achieved.
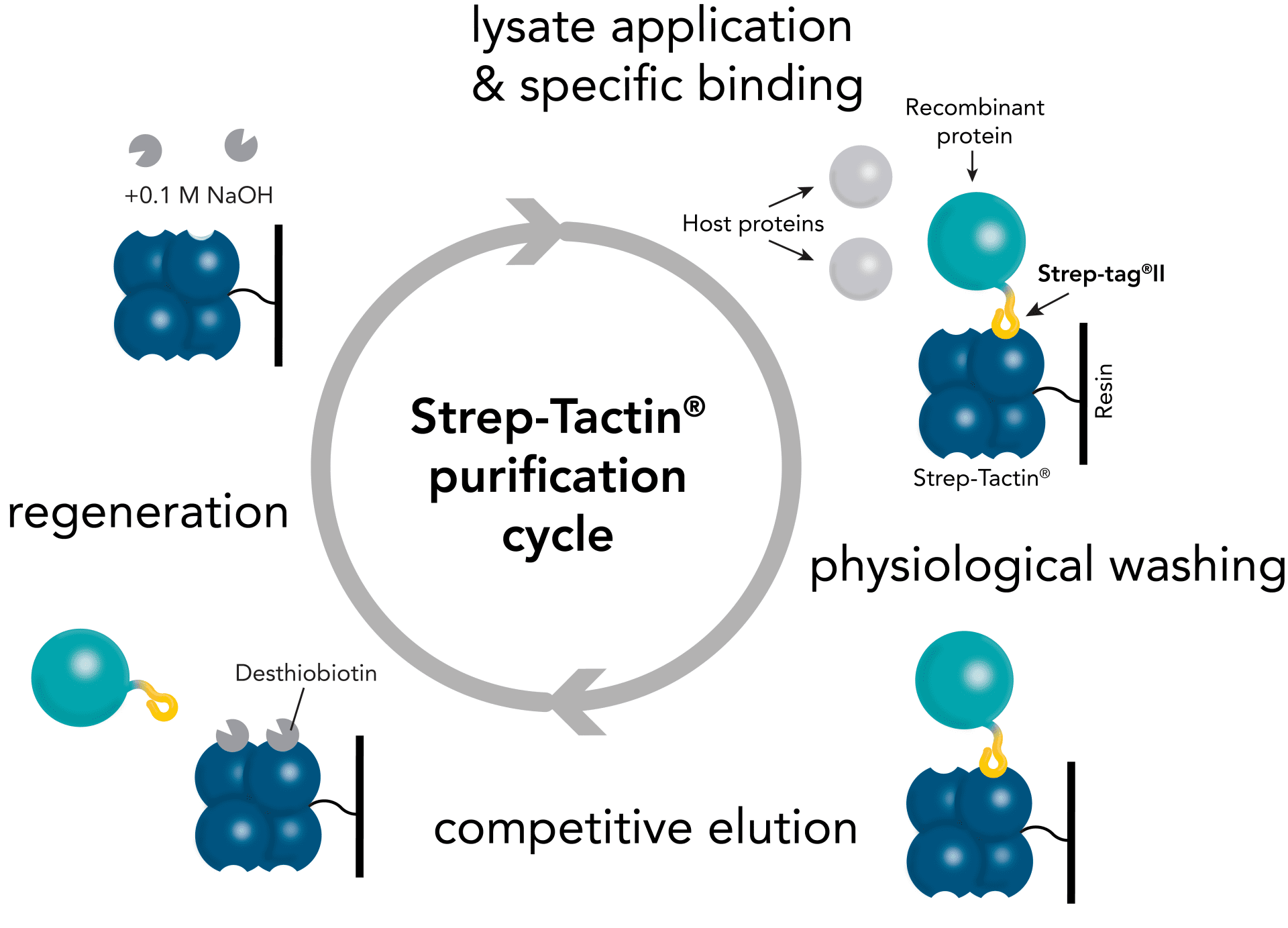
Fig. 3: Protein purification with Strep-Tactin® 4Flow® high capacity is performed with our ususal wash (Buffer W) and elution buffers (Buffer E) for Strep-Tactin® resins. The only protocol adaptation is our recommendation for regeneration: this should be done with 0.1 M NaOH. However, our HABA-containing Buffer R can still be used for functionality tests as before.
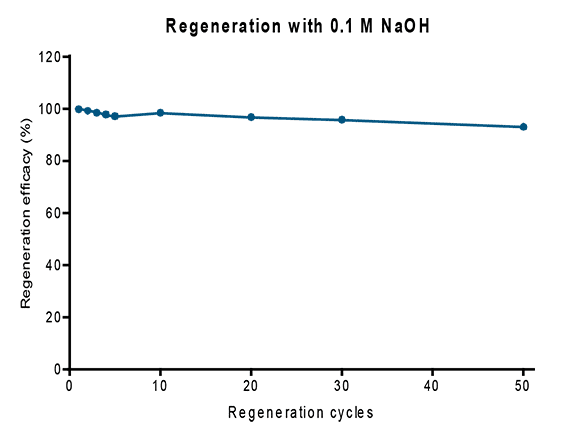
Fig. 4: Strep-Tactin® 4Flow® high capacity was regenerated with 0.1 M NaOH (1 ml FPLC columns). Protein binding capacity of a nanobody fused to a Twin-Strep-tag® remained stable over at least 50 cycles.