Green fluorescent protein (GFP) was isolated from the bioluminescent jellyfish Aequorea victoria by Osamu Shimomura in 1962. It wasn’t until 1994 that Martin Chalfie first applied it to monitor gene expression. The utility of GFP as a tool soon became apparent, and a raft of other useful fluorescent proteins in a rainbow of colors were isolated and developed. The value of GFP was later recognized in the 2008 Nobel Prize. Fluorescent proteins are invaluable in many aspects of life science. For example, their fusions/chimeras enable the detection and quantification of protein/gene expression and the characterization of molecular interactions. Jackson ImmunoResearch now offers polyclonal Rabbit Anti-GFP antibody conjugates for the detection of GFP and its derivatives across a range of applications.
AffiniPure™ Rabbit Anti-GFP antibody
Introduction
GFP is a common protein tag that can be used to generate fusion proteins in a variety of prokaryotic and eukaryotic systems. It can be expressed either transiently or constitutively depending on the researcher’s goal. This tag is useful as a reporter molecule because it does not require exogenous substrates or cofactors to generate fluorescence (citation). GFP is employed in numerous applications including quantification of gene expression, protein localization within a living organism, studying protein interactions, and as a biosensor.
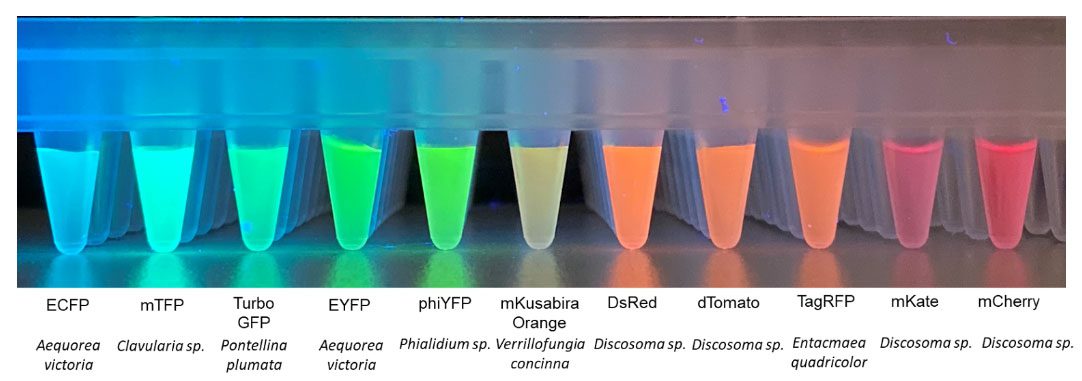
Figure 1: GFP is one of many fluorescent proteins characterized from different aquatic species.
Developed by the laboratories of Thastrup and Falkow in the late nineties, enhanced GFP (EGFP) possesses a single point mutation (F64L) that improves the brightness and subsequent tractability of the molecule. EGFP is the most prevalent variant of GFP used in molecular biology currently. Other variants of the initial GFP have subsequently been developed that have different spectral characteristics, giving rise to yellow and blue (cyan) fluorescent proteins.
After Shimomura’s discovery of GFP, scientists began to investigate the properties of fluorescent proteins from other organisms. These genetically encoded fluorescent reporters were also subject to modification to alter their spectral characteristics so that they would excite and emit at different wavelengths, as seen in Fig 1.
Anti-GFP antibodies allow the detection of the GFP protein when direct fluorescence detection is not possible. Examples include autofluorescence in the GFP channel, when another reporter molecule is required (Western blotting), or when the fluorescence of the native GFP is compromised or not sufficient and needs to be amplified.
About Jackson ImmunoResearch Anti-GFP
Specificity for avGFP and its derivatives
Jackson ImmunoResearch AffiniPure™ Rabbit Anti-GFP is an affinity-purified polyclonal antibody raised in rabbit and can be used to detect Aequorea victoria Green Fluorescent Protein (avGFP) and its derivatives, including EGFP, ECFP, and EYFP (Fig 2).
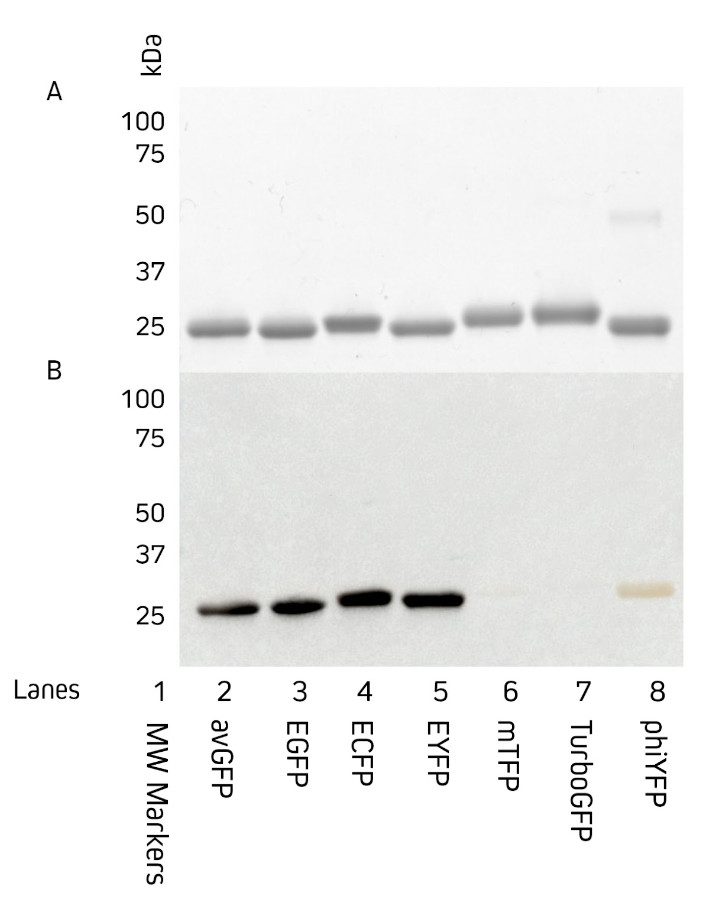
Figure 2: Western blot showing detection of common green fluorescent protein variants. 3 µg of purified protein was loaded onto two 12% acrylamide gels and run in tandem under reducing and denaturing conditions. The first gel, which was stained with Coomassie (A), shows the migration of proteins to approximately 27kDa. The second gel (B) was used for Western Blot. This gel was transferred by electroblotting to a nitrocellulose membrane and probed with a 1:100,000 dilution of AffiniPure™ HRP conjugated Rabbit Anti-GFP (300-035-245).
Imaging with Anti-GFP
Conjugates for immunofluorescence
Jackson ImmunoResearch Anti-GFP antibody is available conjugated to a range of reporter molecules. Image with fluorescence microscopy using JIR’s comprehensive selection of Alexa Fluor® dye conjugates. These conjugates combined with the polyclonal format of JIR Anti-GFP antibodies deliver bright target signal and spectacular signal amplification.
Anti-GFP antibody conjugates can add versatility to an assay when trying to work around autofluorescence or equipment limitations, such as when a laser line or channel is occupied by another target. Using a fluorophore that emits in another channel, Anti-GFP antibodies can circumvent these limitations – making them useful when detecting multiple targets in the same experiment.
Versatile detection of the GFP protein across a range of applications
An Anti-GFP antibody can be used to amplify the signal from GFP expressed by cells in IHC or IF experiments. Anti-GFP may also be used in immunoprecipitation/protein pulldown assays. Our rabbit polyclonal Anti-GFP antibody may be used in ELISA, IHC, ICC, IP, Flow cytometry, and WB.
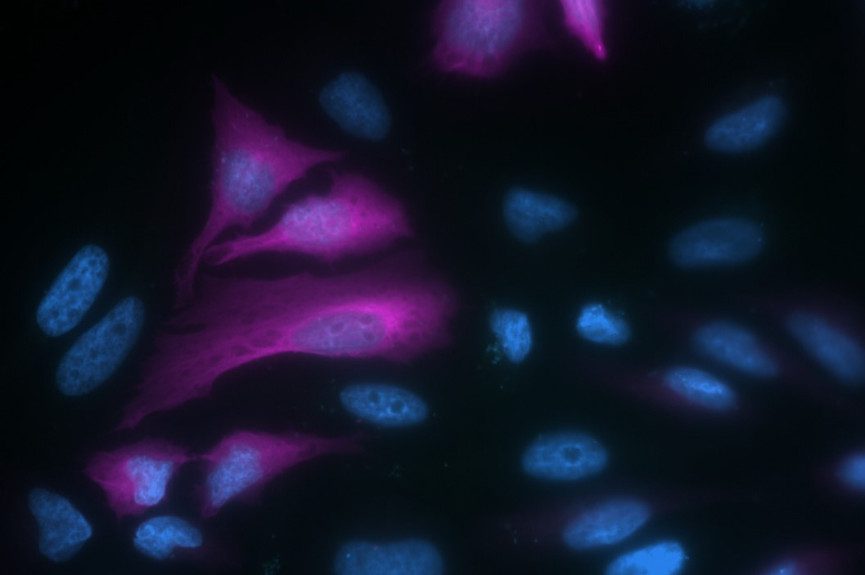
Figure 3: Immunofluorescence microscopy of Hela cells expressing a transiently transfected GFP fusion protein. Alexa Fluor® 647 Rabbit Anti-GFP detection of EGFP tagged Tubulin protein.
| Conjugate | Excitation Peak (nm) | Emission Peak (nm) |
| Alexa Fluor® 488 | 493 | 519 |
| Alexa Fluor® 555 | 552 | 572 |
| R-Phycoerythrin, R-PE | many, 488 | 580 |
| Alexa Fluor® 568 | 577 | 602 |
| Alexa Fluor® 594 | 591 | 614 |
| Alexa Fluor® 647 | 651 | 667 |
| Alexa Fluor® 680 | 684 | 702 |
| Alexa Fluor® 790 | 792 | 803 |
Table 1: Anti-GFP Fluorescent dye conjugates available
Conjugates for IHC
Anti-GFP conjugates also include popular reporter enzymes, Horseradish peroxidase (HRP), and Alkaline phosphatase, as well as a biotin conjugate facilitating the use of chromogenic IHC staining techniques and amplification protocols such as ABC (Avidin-Biotin-Complex) and LSAB (Labeled streptavidin-biotin). Switching to a non-fluorescent detection system enables the use of traditional light-microscopy techniques such as chromogenic IHC.
| Product Description | Product Code |
| Peroxidase AffiniPure™ Rabbit Anti-GFP | 300-035-245 |
| Alkaline Phosphatase AffiniPure™ Rabbit Anti-GFP | 300-055-245 |
| Biotin-SP (long spacer) AffiniPure™ Rabbit Anti-GFP | 300-065-245 |
Protein expression: qualification and quantification
Western Blot and ELISA (Enzyme-linked immunosorbent assays) can be used to both confirm and quantitate the expression of proteins. GFP is a popular reporter molecule used to identify successful protein expression in the absence of protein-specific antibodies. Read our article exploring the use of JIR Anti-GFP antibodies to assess protein expression qualitatively and quantitatively.
Detection of GFP by Flow Cytometry: Case study
Flow cytometry is a useful technique for screening and quantifying expression, particularly when proteins are expressed on the surface of a cell. The GFP tag is frequently used as a reporter molecule to track protein expression by flow cytometry. Learn how Jackson ImmunoResearch AffiniPure™ Rabbit Anti-GFP can be used to detect a GFP fusion protein expressed on yeast cells by flow cytometry.
Anti-GFP Antibodies and conjugates available from Jackson ImmunoResearch
| Product Description | Conjugate | Product Code |
| AffiniPure™ Rabbit Anti-GFP | Unconjugated | 300-005-245 |
| Peroxidase AffiniPure™ Rabbit Anti-GFP | Horseradish Peroxidase | 300-035-245 |
| Alkaline Phosphatase AffiniPure™ Rabbit Anti-GFP | Alkaline Phosphatase | 300-055-245 |
| Biotin-SP (long spacer) AffiniPure™ Rabbit Anti-GFP | Biotin-SP (long spacer) | 300-065-245 |
| Alexa Fluor® 488 AffiniPure™ Rabbit Anti-GFP | Alexa Fluor® 488 | 300-545-245 |
| R-Phycoerythrin AffiniPure™ Rabbit Anti-GFP | R-Phycoerythrin | 300-115-245 |
| Alexa Fluor® 555 AffiniPure™ Rabbit Anti-GFP | Alexa Fluor® 555 | 300-565-245 |
| Alexa Fluor® 568 AffiniPure™ Rabbit Anti-GFP | Alexa Fluor® 568 | 300-575-245 |
| Alexa Fluor® 594 AffiniPure™ Rabbit Anti-GFP | Alexa Fluor® 594 | 300-585-245 |
| Alexa Fluor® 647 AffiniPure™ Rabbit Anti-GFP | Alexa Fluor® 647 | 300-605-245 |
| Alexa Fluor® 680 AffiniPure™ Rabbit Anti-GFP | Alexa Fluor® 680 | 300-625-245 |
| Alexa Fluor® 790 AffiniPure™ Rabbit Anti-GFP | Alexa Fluor® 790 | 300-655-245 |
Table 2: Anti-GFP antibody conjugates
References
- Cormack, B. P., Valdivia, R. H., & Falkow, S. (1996). FACS-optimized mutants of the green fluorescent protein (GFP). Gene, 173(1 Spec No), 33-38. https://doi.org/10.1016/0378-1119(95)00685-0
- Chalfie, M., Tu, Y., Euskirchen, G., Ward, W. W., & Prasher, D. C. (1994). Green fluorescent protein as a marker for gene expression. Science (New York, N.Y.), 263(5148), 802-805. https://doi.org/10.1126/science.8303295
- Chalfie, M. (1995), GREEN FLUORESCENT PROTEIN. Photochemistry and Photobiology, 62: 651-656. https://doi.org/10.1111/j.1751-1097.1995.tb08712.x
- Falkow, S., Valdivia, R., Cormack, B.,(1996). FACS-optimized mutants of the green fluorescent protein (GFP). Gene,173, 1, 33-38, https://doi.org/10.1016/0378-1119(95)00685-0.
- Kusser, K. L., & Randall, T. D. (2003). Simultaneous detection of EGFP and cell surface markers by fluorescence microscopy in lymphoid tissues. The journal of histochemistry and cytochemistry : official journal of the Histochemistry Society, 51 (1), 5-14. https://doi.org/10.1177/002215540305100102
- Lippincott-Schwartz, J., Snapp, E., & Kenworthy, A. (2001). Studying protein dynamics in living cells. Nature reviews. Molecular cell biology, 2(6), 444-456. https://doi.org/10.1038/35073068
- Scandella, V., Paolicelli, R. C., & Knobloch, M. (2020). A novel protocol to detect green fluorescent protein in unfixed, snap-frozen tissue. Scientific reports, 10(1), 14642. https://doi.org/10.1038/s41598-020-71493-x
- SHIMOMURA, O., JOHNSON, F. H., & SAIGA, Y. (1962). Extraction, purification and properties of aequorin, a bioluminescent protein from the luminous hydromedusan, Aequorea. Journal of cellular and comparative physiology, 59, 223-239. https://doi.org/10.1002/jcp.1030590302
- Snapp E. (2005). Design and use of fluorescent fusion proteins in cell biology. Current protocols in cell biology, Chapter 21, 21.4.1-21.4.13. https://doi.org/10.1002/0471143030.cb2104s27
- Soboleski, M. R., Oaks, J., & Halford, W. P. (2005). Green fluorescent protein is a quantitative reporter of gene expression in individual eukaryotic cells. FASEB journal : official publication of the Federation of American Societies for Experimental Biology, 19(3), 440-442. https://doi.org/10.1096/fj.04-3180fje
- Stretton, S., Techkarnjanaruk, S., McLennan, A. M., & Goodman, A. E. (1998). Use of green fluorescent protein to tag and investigate gene expression in marine bacteria. Applied and environmental microbiology, 64(7), 2554-2559. https://doi.org/10.1128/AEM.64.7.2554-2559.1998
- Subramanian, S., & Srienc, F. (1996). Quantitative analysis of transient gene expression in mammalian cells using the green fluorescent protein. Journal of biotechnology, 49(1-3), 137-151. https://doi.org/10.1016/0168-1656(96)01536-2
- Tsien R. Y. (1998). The green fluorescent protein. Annual review of biochemistry, 67, 509-544. https://doi.org/10.1146/annurev.biochem.67.1.509