Selection and Location of Affinity-Purified Secondary Antibodies
Step 1. Affinity-purified secondary antibodies are offered in three different forms. Select from Whole IgG, F(ab’)2 fragment, or Fab fragment antibodies.
Whole IgG antibodies are isolated as intact molecules from antisera by immunoaffinity chromatography. They have an Fc portion and two antigen binding Fab portions joined together by disulphide bonds (Figure 1) and therefore they are divalent. The average molecular weight is reported to be about 160 kDa. The whole IgG form of antibodies is suitable for the majority of immunodetection procedures and is the most cost effective.
F(ab’)2 fragment antibodies are generated by pepsin digestion of whole IgG antibodies to remove most of the Fc region while leaving intact some of the hinge region. F(ab’)2 fragments have two antigen‑binding Fab portions linked together by disulphide bonds and therefore they are divalent. The average molecular weight is about 110 kDa. They are used for specific applications, such as to avoid binding of secondary antibodies to live cells with Fc receptors or to Protein A or Protein G.
However, binding of primary antibodies to Fc receptors also may occur if they are whole IgG antibodies, creating background regardless of the form of the secondary antibody. To block whole IgG primary and secondary antibodies from binding to Fc receptors, incubate cells in buffer containing 5% normal serum from the host species of the labelled secondary antibody. To prevent capping, endocytosis and regeneration of Fc receptors on living cells, incubate at 4°C in buffer containing 5% normal serum with sodium azide added to inhibit metabolism.
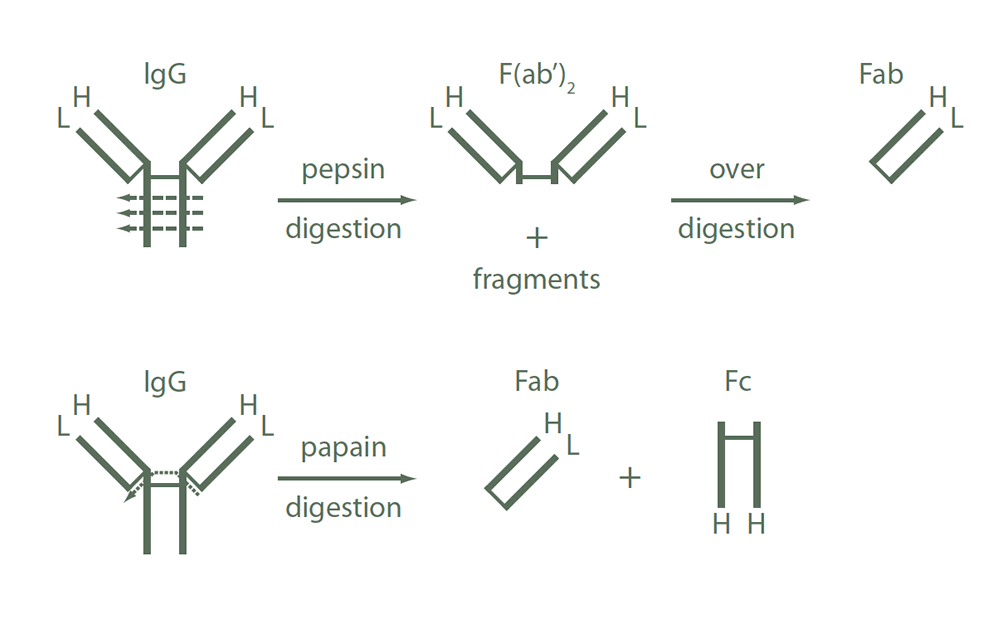
Figure 1. Schematic representation of IgG fragments generated by enzymatic digestions.
Caution: Never block with normal serum or IgG from the host species of the primary antibody. If immunoglobulins in normal serum bind to the specimen of interest, they will be recognised by the labelled secondary antibody, resulting in higher background.
Bovine serum albumin (BSA) and dry milk, both commonly used for blocking, may contain bovine IgG. With the exception of Bovine Anti‑Goat IgG, many secondary antibodies such as Anti‑Bovine, Anti‑Goat and Anti‑Sheep will react strongly with bovine IgG. Therefore, use of BSA or dry milk for blocking or diluting these antibodies may significantly increase background and/or reduce antibody titre. For blocking, use normal serum (5% v/v) from the host species of the labelled secondary antibody.
Fab fragment antibodies are generated by papain digestion of whole IgG antibodies to remove the entire Fc portion, including the hinge region (Figure 1). These antibodies are monovalent, containing only a single antigen binding site. The molecular weight of Fab fragments is about 50 kDa. They can be used to block endogenous immunoglobulins on cells, tissues, or other surfaces, and to block the exposed immunoglobulins in multiple labelling experiments using primary antibodies from the same species.
In contrast, divalent (whole IgG or F(ab’)2 fragment) antibodies should not be used for blocking since they have two binding sites. After blocking, some of the binding sites would be available to capture the primary antibody introduced in a subsequent step, resulting in higher background and/or coincidental labelling.
Step 2. Select the secondary antibody.
The antibodies are listed alphabetically according to the host species of the primary antibody. For example, if the primary antibody is made in mouse, go to the “Anti‑Mouse” section.
Note: Both anti‑Syrian and anti‑Armenian hamster secondary antibodies are listed under “Anti‑Hamster”. It is important to know in which strain of hamster the primary antibody was produced since cross‑reaction between the strains is not complete.
Step 3. Select the host species of the secondary antibody.
Selection of the host species for a secondary antibody involves many considerations, including but not limited to: 1) Antibodies from some host species may not be adsorbed against cross‑reacting species of interest. Choose a host species with the required adsorptions. 2) Host species compatibility. Some host species may not be compatible with other species in multiple‑labelling experiments. In general, all secondary antibodies for multiple labelling should come from the same host species. 3) Binding to Protein A and Protein G. Rabbit antibodies bind well to Protein A and Protein G, but goat and donkey antibodies bind better to Protein G. 4) Personal preference or experience. In our experience there appears to be no host species‑specific difference in the quality of secondary antibodies.
Step 4. Select the secondary antibody specificity under “Antibody Description”.
The following explanations of terms may assist in selecting the most appropriate antibody specificity.
Note: Immunoglobulins from different species share similar structures, with similarities being related to closeness in phylogeny. Antibodies against immunoglobulins from one species may cross‑react with a number of other species unless they have been specifically adsorbed against the cross‑reacting species. Antibodies that have been adsorbed against other species will contain “(min X…Sr Prot)” in the antibody description.
Anti‑IgG (H+L)
These antibodies react with both the heavy and light chains of the IgG molecule, i.e. with both the Fc and F(ab’)2 / Fab portions of IgG (Figure 1). Anti‑IgG (H+L) antibodies also react with other immunoglobulin classes (IgM, IgA, IgD, IgE) and subclasses since they all share the same light chains (either kappa or lambda). Anti‑IgG (H+L) antibodies have broader epitope recognition than anti‑fragment specific antibodies. They are suggested for all general immunodetection procedures.
Anti‑IgG, Fc/Fcγ fragment specific
These antibodies react with the Fc portion of the IgG heavy chain. They have been tested by ELISA and/or adsorbed against Fab fragments. In some cases, they are additionally tested and/or adsorbed to minimise cross‑reactivity to IgM and/or IgA. In such cases (anti‑human, anti‑mouse, and anti‑rat), they are labelled “Anti‑IgG, Fcγ”.
Caution: Anti‑IgG, Fcγ fragment specific antibodies may not react equally with all monoclonal primary antibodies. For an anti‑mouse IgG, Fcγ fragment specific antibody with balanced reactivity to four subclasses of IgG, select goat anti‑mouse IgG (subclasses 1+2a+2b+3), Fcγ fragment specific (min X Hu, Bov, Rb Sr Prot).
Anti‑Mouse IgG, Fcγ subclass specific
These antibodies react with the Fc portion of the heavy chain of individual subclasses of mouse IgG. They have been tested by ELISA and adsorbed to minimise cross‑reactivity to other subclasses, Fab fragments, IgM, and a few other species of IgG. Anti‑Mouse IgG, Fcγ subclass specific antibodies react with individual subclasses of mouse IgG. They are intended for distinguishing between different subclasses of mouse IgG primary antibodies in multiple labelling experiments, or for IgG subclass determination.
Anti‑IgG, F(ab’)2 fragment specific
These antibodies react with the F(ab’)2 / Fab portion of IgG. They have been tested by ELISA and/or adsorbed against Fc fragments. They are not specific for IgG since they react with light chains, and therefore also react with other immunoglobulin classes (IgA, IgM, IgD, and IgE) and subclasses sharing the same light chains.
(min X … Sr Prot)
Secondary antibodies against one species may cross‑react with other species unless they have been specifically adsorbed against the other species. Antibodies with “(min X … Sr Prot)” in the description have been tested and/or adsorbed against IgG and/or serum proteins of those species indicated in the parentheses. They are recommended when the presence of immunoglobulins from other species may lead to interfering cross‑reactivities. However, caution should be exercised when considering antibodies that have been adsorbed against closely related species, since they have greatly reduced epitope recognition and may recognise some monoclonals poorly. For example, only use anti‑mouse IgG adsorbed against rat IgG to detect a mouse primary antibody in rat tissue which contains endogenous rat immunoglobulins, or in a multiple labelling application which includes a rat primary antibody. Use anti‑mouse IgG not adsorbed against rat IgG to detect a mouse primary antibody in the absence of rat immunoglobulins. Two other examples of antibodies which have diminished epitope recognition after adsorption with closely related species are Anti‑Rat IgG (min X … Mouse … Sr Prot) and Anti‑Armenian Hamster IgG (min X … Mouse, Rat … Sr Prot). Refer to “ML (Multiple Labelling)” for further information.
The following abbreviations are used in the parentheses:
Bov = Bovine
Ck = Chicken
Gt = Goat
GP = Guinea Pig
Ar Hms = Armenian Hamster
Sy Hms = Syrian Hamster
Hrs = Horse
Ms = Mouse
Rb = Rabbit
Shp = Sheep
Sw = Swine
Sr = Serum
Prot = Protein
ML (multiple labelling) Some antibodies are designated “ML” to emphasise their usefulness in multiple labelling in addition to single labelling. For further information see: “Multiple Labelling (ML) Using Labelled Secondary Antibodies”.
Anti‑Armenian Hamster IgG vs. Anti‑Syrian Hamster IgG
Most hamster monoclonal antibodies are derived from Armenian hamster spleen cell‑mouse myeloma hybridomas. The IgG produced by these hybridomas is Armenian (not Syrian) hamster IgG. Most commercially available polyclonal anti‑hamster IgG antibodies have been anti‑Syrian hamster IgG, which are not as effective as anti‑Armenian hamster IgG in detecting Armenian hamster IgG monoclonal antibodies.
Caution: Anti‑Armenian hamster IgG (H+L) (min X Bov, Hu, Ms, Rb, Rat Sr Prot) may not recognise all Armenian hamster monoclonal antibodies, since it has been adsorbed against closely related species (in bold). Therefore, it is better to use an antibody adsorbed against fewer species, such as Anti‑Armenian Hamster IgG (H+L) (min X Bov Sr Prot), except in those cases where Armenian hamster monoclonals need to be detected in the presence of mouse and/or rat immunoglobulins.
Step 5. Find the desired probe from of the selected antibody by using the filer on the left hand side of Stratech search.
To select the antibody you require visit Jackson ImmunoRsearch’s Specific Search and use the left hand filter system to select the host, target, specificity and other criteria you require. Alternatively use the Advance Search Option (help) to do your search.
Step 6. Complete product description for ordering purposes.
For a complete description of the product, please use the following format to avoid mistakes when placing an order. For example, a product with the code number 115‑096‑072 should be described as follows:
A. Description of the probe, if it is conjugated. If unconjugated, nothing is required here.
B. AffiniPure is our trade name for antibodies which have been isolated from antisera by immunoaffinity chromatography using antigens coupled to agarose beads.
C. Form of the antibody – whole IgG, F(ab’)2 fragment, or Fab fragment antibody.
D. Name of the host species of the secondary antibody.
E. Name of the species with which the antibody reacts.
F. Description of the antibody specificity.
G. List of species against which the antibody has been adsorbed to minimise cross‑reactivity.
Multiple Labelling (ML) Using Labelled Secondary Antibodies
Selection of antibodies for simultaneous detection of more than one antigen depends on at least two important criteria: 1. Availability of secondary antibodies that do not recognise (a) one another (are derived from the same host species), (b) other primary antibodies used in the assay system, (c) immunoglobulins from other species present in the assay system, or (d) endogenous immunoglobulins present in the tissues or cells under investigation. 2. Use of probes (enzyme‑reaction products, fluorophores, or electron‑dense particles) that are well resolved.
The affinity‑purified antibodies marked “ML” (multiple labelling) have been specifically prepared to meet these criteria. One of many possible multiple‑labelling protocols using these reagents is shown in the following example.
| Mouse Tissue Antigen A | Mouse Tissue Antigen B | Mouse Tissue Antigen C |
| Step 1: 5% N. Donkey Serum to Block | Step 4: 5% N. Donkey Serum to Block (if needed) | Step 7: 5% N. Donkey Serum to Block (if needed) |
| Step 2: Goat Anti Antigen A | Step 5: Rabbit Anti Antigen B | Step 8: Rat Anti Antigen C |
| Step 3: Probe I conjugated Donkey Anti‑Goat IgG (H+L)(min X Ck, GP, Sy Hms, Hrs, Hu, Ms, Rb, Rat Sr Prot) | Step 6: Probe II conjugated Donkey Anti‑Rabbit IgG (H+L) (min X Bov, Ck, Gt, GP, Sy Hms, Hrs, Hu, Ms, Rat, Shp Sr Prot) | Step 9: Probe III conjugated Donkey Anti‑Rat IgG (H+L)(min X Bov, Ck, Gt, GP, Sy Hms, Hrs, Hu, Ms, Rb, Shp Sr Prot) |
Note: Wash thoroughly after each step, including after blocking at step 1. With heavy or persistent background further blocking may be required at Steps 4 and 7. Do not dilute any antibody with normal serum or mix antibodies together to save time, which may result in immune complex formation which could increase background.
In this example, the secondary antibodies used in Steps 3, 6, and 9 do not recognise each other since they are all made in donkey. They have been solid‑phase adsorbed so that they do not recognise the other primary antibodies used in Steps 2, 5, and 8. Also, they do not react with endogenous mouse Ig, which may be present in the mouse tissue. For a review of multi‑colour immunofluorescence labelling with confocal microscopy see Brelje, Wessendorf, and Sorenson, “Multi‑colour laser scanning confocal immunofluorescence microscopy: Practical application and limitations.” In Cell Biological Applications of Confocal Microscopy (Methods in Cell Biology. vol. 38). Ed. B. Matsumoto. Orlando, FL: Academic Press, Inc. 1993, pp. 98‑181.