Green fluorescent protein (GFP) and its derivatives are commonly used tags in fusion proteins. They enable visualization and characterization of protein localization and interactions using a variety of imaging techniques. Read more to find out how Jackson ImmunoResearch’s NEW AffiniPure™ Rabbit Anti-GFP, can be used for both immunofluorescence microscopy and chromogenic IHC.
Imaging with Anti-GFP Antibodies
Introduction
Green Fluorescent Protein (GFP) is a commonly used reporter protein that has useful characteristics when it is used to create a fusion protein, for example, visualizing protein localization, detecting and quantifying expression of transgenic proteins in vivo, and for characterizing physiological processes. GFP is maximally excited at 488 nm, which corresponds to the FITC laser line, and is optimally detected at 510 nm, making it detectable with typical lab equipment. An improved version of GFP is EGFP (Enhanced Green Fluorescent Protein), which is regularly used in fusion proteins as both an expression marker and a reporter molecule. Although GFP and its descendants are robust and tractable molecules, the effects of sample processing for microscopy techniques can damage or render it unsuitable under certain conditions. Anti-GFP antibodies allow researchers to continue to use GFP protein fusions and bypass their limitations when conditions lead to their suboptimal performance.
Summary of the benefits of using an Anti-GFP antibody
- Retrieve/rescue and amplify GFP signal.
- Image GFP in tissue samples fixed with formalin that can abrogate GFP luminescence to improve sample integrity/ prevent movement of target proteins in sample.
- Switch the channel to circumvent background/ endogenous GFP signal and autofluorescence.
- Fluorescence flexibility – choose dyes to accommodate experimental needs and equipment
- Switching to reporter enzyme conjugate allows imaging using a non-fluorescent imaging system.
- Probe for GFP in the absence of other expression/purification probes (such as His-Tag or Flag-Tag).
- Enables detection of target protein by a universal tag when antibodies against the target protein are unavailable, unreliable or not economic.
Overcome the Limitations of GFP and its derivatives using Anti-GFP Antibodies
Image successfully even when conditions prevent optimal fluorescence
Like many proteins, GFP requires the conditions around it to be correct in order to be optimally functional. For GFP to fluoresce, it must form a fully folded beta-barrel structure, followed by an intramolecular reaction that requires oxygen to generate the active chromophore (Tsien, 1998). Consequently, GFP is non-fluorescent under anaerobic conditions such as those induced by FFPE (Chia et al., 2019). FFPE and other fixing techniques are often used to prevent the migration of proteins, which can prevent accurate protein localization (Chalfie and Kain., 2005; Morris et al., 2010). However, the process can functionally alter the GFP protein, weakening and destroying the protein’s ability to fluoresce (Kusser et al., 2003).
Another limitation of GFP that prevents its functionality is its sensitivity to acid, which may preclude its use when visualizing certain cellular organelles (Shinoda et al., 2018). GFP’s chromophore can only absorb and emit light in its protonated state (Tsien, 1998). Therefore, at pHs below its pKa (<pH 6.0), where the chromophore is present in its deprotonated state, the efficiency of the protein to fluoresce is reduced.
JIR Anti-GFP antibodies offer robust detection of GFP, recognizing GFP in native or fixed/denatured conditions as long as the protein is not degraded, enabling the GFP tag to be used in circumstances where the protein would not be functional. The polyclonal format of JIR Rabbit Anti-GFP enables the binding of multiple antibodies to the target, bringing additional fluorescent molecules, thus enhancing the GFP signal beyond the output associated with GFP alone. This is demonstrated in Figure 1, where HEK 293T cells transfected with EGFP are probed with Alexa Fluor® 488 Rabbit Anti-GFP. We can observe the amplification of the GFP signal as the concentration of Rabbit Anti-GFP increases.

Figure 1: GFP signal can be poor and difficult to visualize (panel B slide 4). Indirect staining using a secondary antibody conjugated to a fluorescent dye can enable signal amplification (panel B, slides 1, 2, 3) while offering the opportunity to utilize other channels. HEK293T cells were seeded at 2×105 cells onto 8-well plates coated with Fibronectin at 2 μg/well. Cells were transfected with or without 200 ng plasmid DNA expressing EGFP, with 2μg PEI / well as transfection agent. Cells were incubated at 37°C, 5% CO2 overnight to allow expression. The following day, cells were fixed with 4% Formaldehyde and immunostained using Alexa Fluor® 488 Rabbit Anti-GFP (300-545-245) as per concentration noted in figure 1 a. All slides are stained by DAPI at 1:50,000 dilution. Slides were mounted with VectaShield and imaged with a Nikon DS-Qi2, Binning: 3.0×3.0, Exposure: 300 ms, Gain: 1.0x
Avoid overlapping signal with autofluorescence and/or background
Autofluorescence is intrinsic fluorescence that occurs due to cellular components or as an artifact of sample processing that generates background signal. It often interferes with the detection and interpretation of genuine signals from target proteins. Autofluorescence may occur across the spectrum but is often observed emitting between 350 and 550 nm, which overlaps with the emission profile of GFP; this can be problematic as the GFP signal cannot be distinguished over the background autofluorescence (figure 2, panel 1a).
Anti-GFP antibodies can be chosen as conjugates to a wide range of fluorophores, enabling researchers to choose an emission profile that does not overlap with the GFP signal or sample autofluorescence.
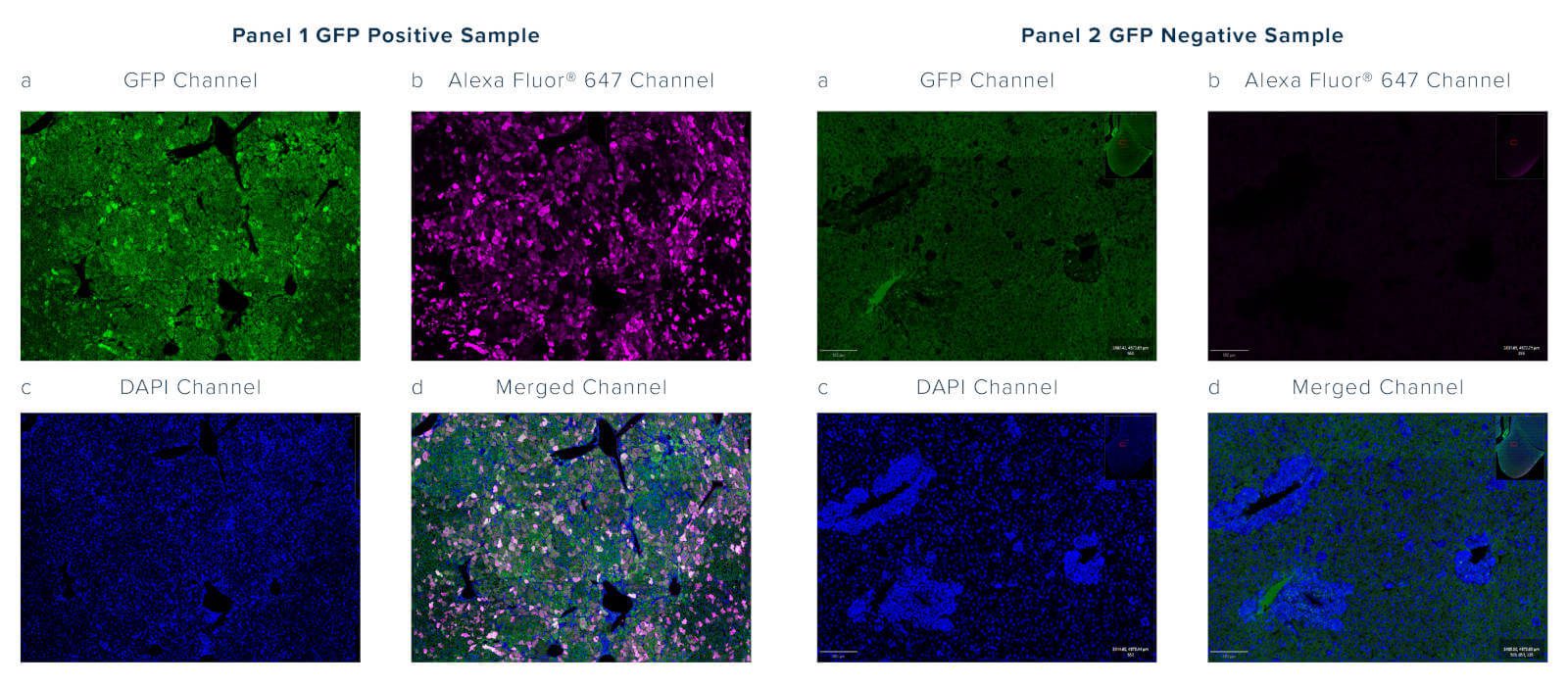
Figure 2: Immunofluorescence microscopy on a transgenic mouse liver constitutively expressing a GFP fusion protein. Panel 1a shows GFP positive tissue with signal abrogated by sample fixing but with profuse autofluorescence observed in the 488nm/green channel (seen in Panel 2a of GFP negative tissue). Genuine GFP signal (red channel) was confirmed by immunostaining samples with Alexa Fluor® 647 Rabbit Anti-GFP (300-605-245) (Panel 1b) but absent in Panel 2b where no GFP is expressed. The merge of green and red channels in Panel 1d highlights the different localization of the two signals, with minimal overlap, allowing autofluorescence to be deconvoluted from the GFP signal.
Conjugates for flexible experimental design
Modern microscopy techniques continue to evolve, often seeking to label multiple targets simultaneously or push resolution boundaries.
Jackson Immunoresearch Anti-GFP is available in a wide range of fluorescent conjugates, providing different emission profiles, so that conjugates can be appropriately selected for the available equipment and requirements of the experiment. Conjugated antibodies may also be chosen to increase panel flexibility, so more targets can be accommodated in a single experiment. When fluors are selected in longer wavelengths, such as Alexa Fluor® 647, signals can be resolved without spectral unmixing, which often requires additional or expensive equipment or data compensation.
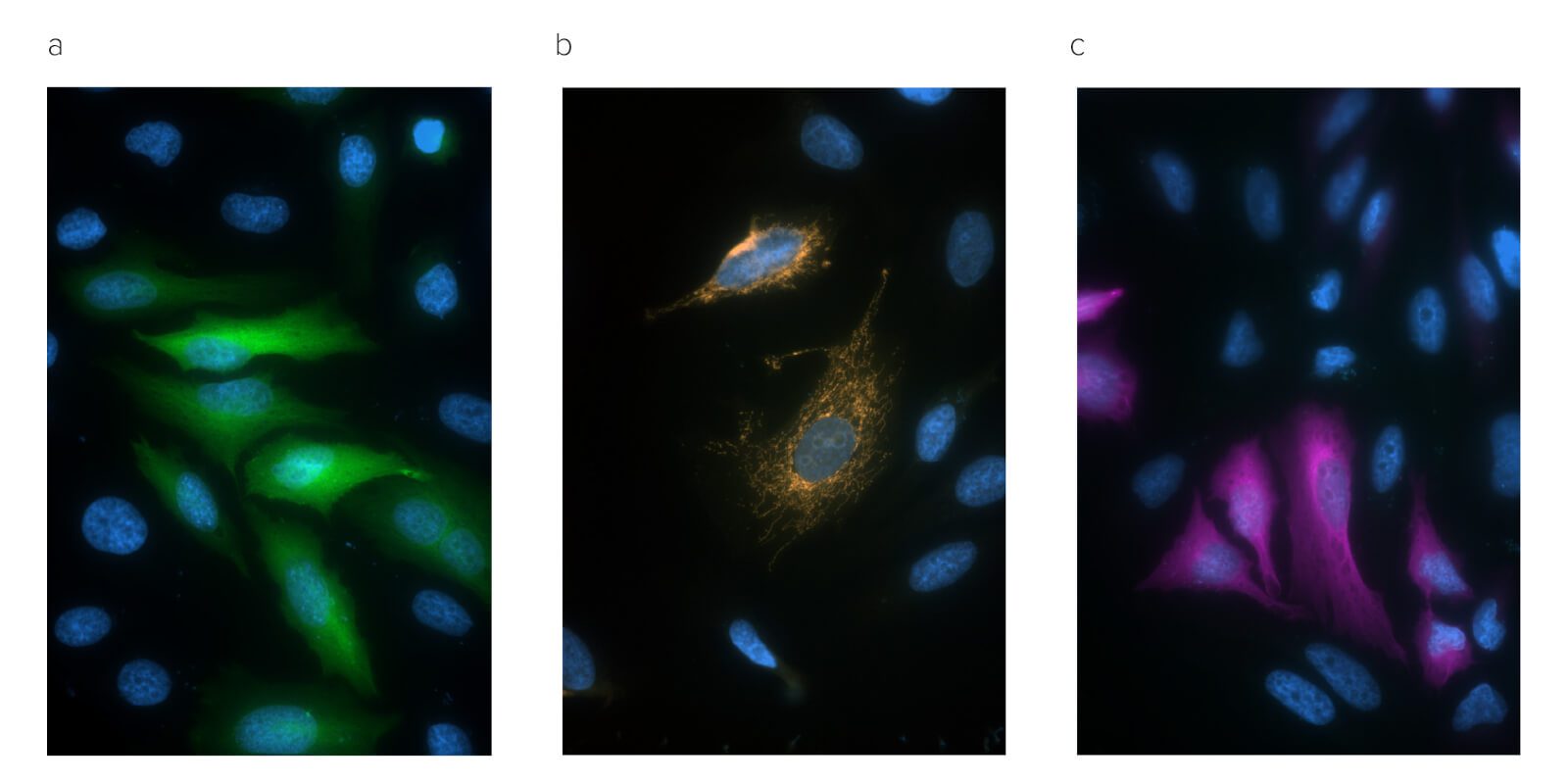
Figure 4: Immunofluorescence microscopy of Hela cells transiently transfected with GFP fusion proteins.
Panel A, Alexa Fluor® 488 Rabbit Anti-GFP detection of EGFP tagged Tubulin protein (300-545-245);
Panel B, Alexa Fluor® 594 Rabbit Anti-GFP detection of EGFP localized to mitochondria (300-585-245);
Panel C, Alexa Fluor® 647 Rabbit Anti-GFP detection of EGFP tagged Tubulin protein (300-605-245).
Conjugated Anti-GFP antibodies can also add versatility to experiments restricted by equipment limitations, such as when fluorescent imaging systems are unavailable or unsuitable. Jackson ImmunoResearch Anti-GFP antibody is available conjugated to reporter enzymes, Horseradish peroxidase (HRP), and Alkaline phosphatase, as well as a biotinylated conjugate facilitating the use of chromogenic IHC staining techniques and amplification protocols such as ABC, LSAB.
Case Study: Anti-GFP antibodies and the IHC experiment
Transgenic animal models are powerful tools for gaining deeper knowledge about protein expression and interactions within an organism. Transproteins are regularly generated as fusions with reporter molecules such as GFP to enable their detection, co-localization, or characterize their interactions using such techniques as FRET (Förster resonance energy transfer) or SRM (Super-resolution microscopy). Chromogenic IHC is an immunohistochemical technique that uses antibodies to target protein antigens, followed by a chromogen/substrate combination to visualize them in tissue samples. Here, we investigate the presence of a constitutively expressed GFP fusion protein in the liver of a transgenic mouse, using direct and indirect immunostaining methods incorporating Jackson ImmunoResearch Rabbit Anti-GFP antibody conjugates.
Direct Immunostaining: Detection of constitutively expressed GFP fusion protein by Peroxidase Rabbit Anti-GFP antibody
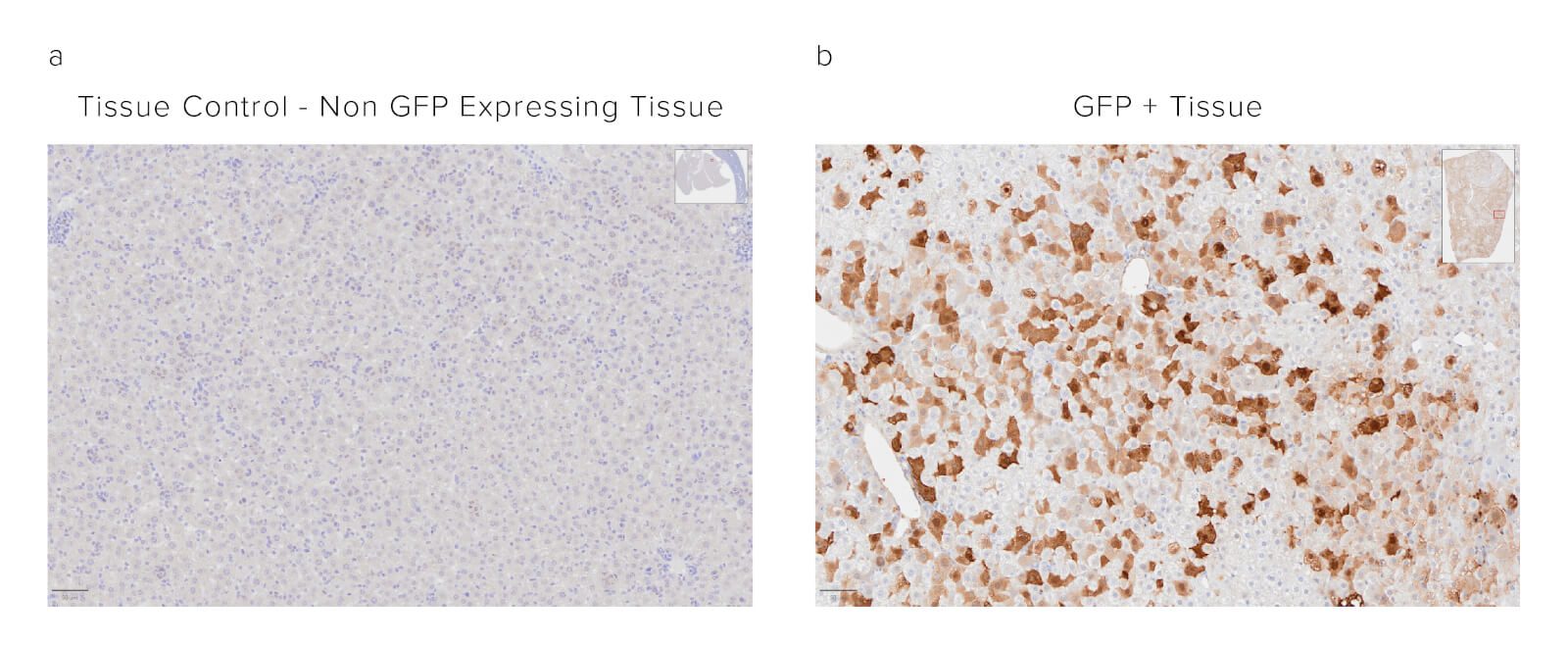
Figure 5: Direct immunostaining of formalin-fixed paraffin-embedded (FFPE) Mouse liver tissue with HRP/DAB staining. Panel A shows the tissue control of non-GFP-expressing tissue. Localization of the GFP protein is observed in Panel B as brown staining synonymous with catalyzation of the DAB substrate by the peroxidase conjugate of the Anti-GFP antibody.
Experimental setup
Formalin-fixed paraffin-embedded (FFPE) mouse liver. The sample was baked, deparaffinized, and hydrated before Heat-Induced Epitope Retrieval (HIER) at 120/90°C, pH 6. Endogenous peroxidase was inactivated with 3% Hydrogen peroxide in dH2O before blocking in 3% BSA in PBS for 1 hr. The sample was incubated with Peroxidase Rabbit Anti-GFP (300-035-245) at a dilution of 1:1000. The sample was washed and incubated with a DAB substrate and imaged at 20x by the Histology Research Core Facility in the Dept. of Cell and Molecular Physiology at University of North Carolina.
Indirect immunostaining: Biotinylated Rabbit Anti-GFP
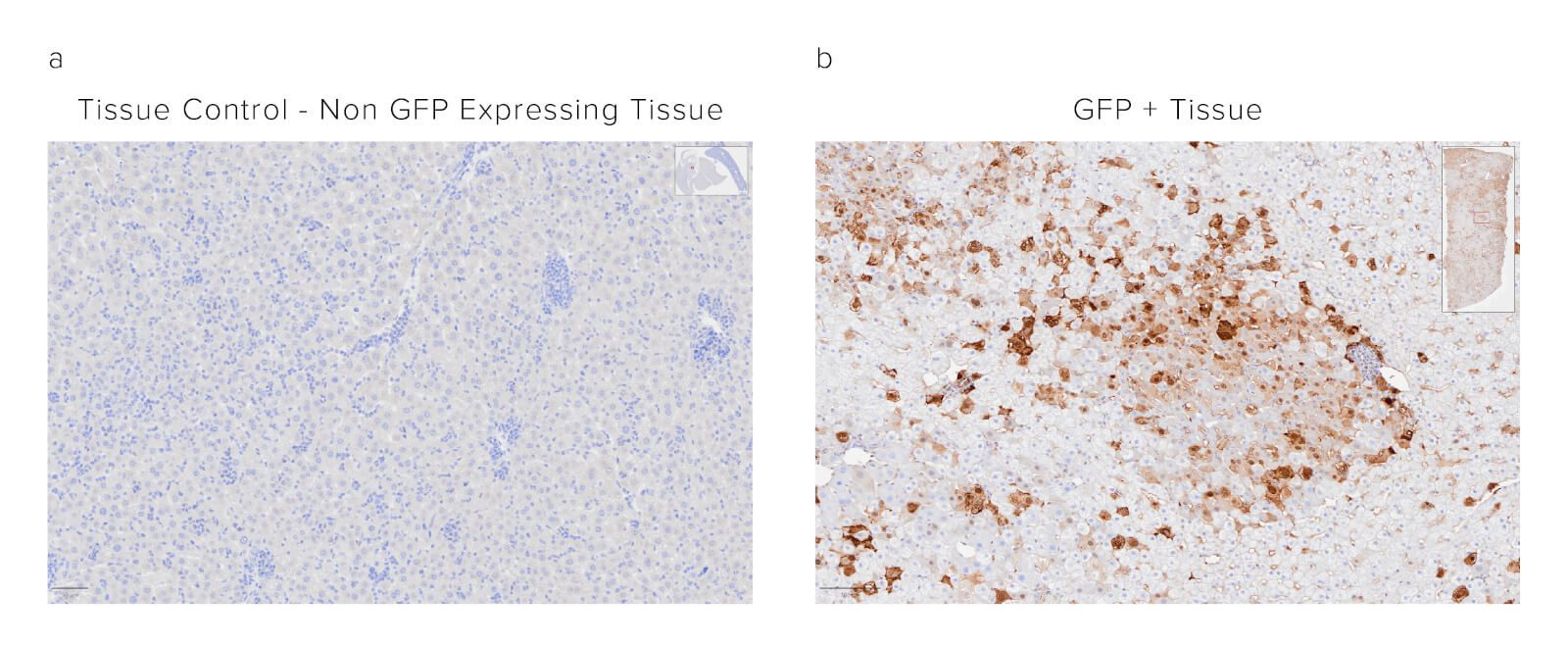
Figure 6: Indirect immunostaining using JIR Biotinylated Rabbit Anti-GFP Antibody (300-065-245) followed by ABC (Avidin Biotin Complex) kit of FFPE mouse liver. Panel A shows the tissue control of non-GFP-expressing tissue. Localization of the GFP protein is observed in Panel B as brown staining synonymous with the catalyzation of the ABC complex.
Experimental setup
Sample: Formalin-fixed paraffin-embedded (FFPE) mouse liver. The sample was baked, deparaffinized, and hydrated before HIER at 120/90°C pH 6. Endogenous peroxidase was inactivated with 3% Hydrogen peroxide in dH2O before blocking in 3% BSA in PBS for 1hr. Sample was then incubated with Biotinylated Rabbit Anti-GFP (300-065-245) at a dilution of 1:1000. Sample was washed and then incubated with ABC kit at 1:50 dilution (Vector) and imaged at 20x by the Histology Research Core Facility in the Dept. of Cell and Molecular Physiology at University of North Carolina.
Conclusion
Chromogenic IHC is a powerful tool for detecting fusion proteins in transgenic animal models. Jackson ImmunoResearch Rabbit Anti-GFP antibody conjugates offer specific and sensitive detection of GFP in FFPE tissue when used with standard chromogenic substrate protocols, or ABC signal amplification methods.
References
- Cormack, B. P., Valdivia, R. H., & Falkow, S. (1996). FACS-optimized mutants of the green fluorescent protein (GFP). Gene, 173(1 Spec No), 33-38. https://doi.org/10.1016/0378-1119(95)00685-0
- Chalfie, M., Tu, Y., Euskirchen, G., Ward, W. W., & Prasher, D. C. (1994). Green fluorescent protein as a marker for gene expression. Science (New York, N.Y.), 263(5148), 802-805. https://doi.org/10.1126/science.8303295
- Chalfie, M. (1995), GREEN FLUORESCENT PROTEIN. Photochemistry and Photobiology, 62: 651-656. https://doi.org/10.1111/j.1751-1097.1995.tb08712.x
- Chia, H.E., Marsh, E.N. and Biteen, J.S. (2019) ‘Extending fluorescence microscopy into anaerobic environments’, Current Opinion in Chemical Biology, 51, pp. 98-104. https://doi.org/10.1016/j.cbpa.2019.05.008.
- Kusser, K. L., & Randall, T. D. (2003). Simultaneous detection of EGFP and cell surface markers by fluorescence microscopy in lymphoid tissues. The journal of histochemistry and cytochemistry : official journal of the Histochemistry Society, 51(1), 5-14. https://doi.org/10.1177/002215540305100102
- Lippincott-Schwartz, J., Snapp, E., & Kenworthy, A. (2001). Studying protein dynamics in living cells. Nature reviews. Molecular cell biology, 2(6), 444-456. https://doi.org/10.1038/35073068
- Scandella, V., Paolicelli, R. C., & Knobloch, M. (2020). A novel protocol to detect green fluorescent protein in unfixed, snap-frozen tissue. Scientific reports, 10(1), 14642. https://doi.org/10.1038/s41598-020-71493-x
- Shinoda H, Shannon M, Nagai T. Fluorescent Proteins for Investigating Biological Events in Acidic Environments. Int J Mol Sci. 2018;19(6):1548. Published 2018 May 23. https://doi.org/10.3390/ijms19061548
- SHIMOMURA, O., JOHNSON, F. H., & SAIGA, Y. (1962). Extraction, purification, and properties of aequorin, a bioluminescent protein from the luminous hydromedusan, Aequorea. Journal of cellular and comparative physiology, 59, 223-239. https://doi.org/10.1002/jcp.1030590302
- Snapp E. (2005). Design and use of fluorescent fusion proteins in cell biology. Current protocols in cell biology, Chapter 21, 21.4.1-21.4.13. https://doi.org/10.1002/0471143030.cb2104s27
- Soboleski, M. R., Oaks, J., & Halford, W. P. (2005). Green fluorescent protein is a quantitative reporter of gene expression in individual eukaryotic cells. FASEB journal : official publication of the Federation of American Societies for Experimental Biology, 19(3), 440-442. https://doi.org/10.1096/fj.04-3180fje
- Stretton, S., Techkarnjanaruk, S., McLennan, A. M., & Goodman, A. E. (1998). Use of green fluorescent protein to tag and investigate gene expression in marine bacteria. Applied and environmental microbiology, 64(7), 2554-2559. https://doi.org/10.1128/AEM.64.7.2554-2559.1998
- Subramanian, S., & Srienc, F. (1996). Quantitative analysis of transient gene expression in mammalian cells using the green fluorescent protein. Journal of Biotechnology, 49(1-3), 137-151. https://doi.org/10.1016/0168-1656(96)01536-2
- Tsien R. Y. (1998). The green fluorescent protein. Annual review of biochemistry, pp. 67, 509-544. https://doi.org/10.1146/annurev.biochem.67.1.509
