Product Groups
Whole IgG Affinity-Purified Secondary Antibodies
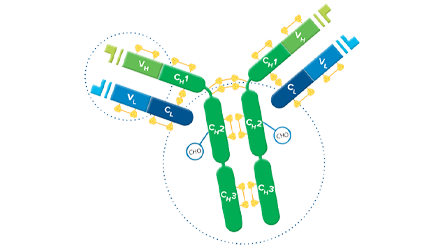
Whole IgG antibodies are suitable for the majority of immunodetection procedures.
F(ab')2 Fragment Affinity-Purified Secondary Antibodies
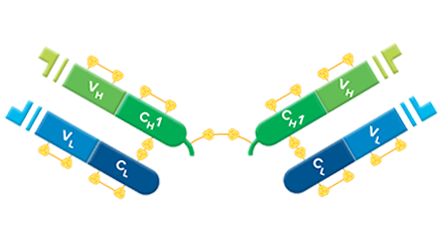
F(ab’)2 fragments are used for specific applications, such as to avoid binding of secondary antibodies to live cells with Fc receptors to Protein A or Protein G.
Monovalent Fab Fragment Affinity-Purified Secondary Antibodies
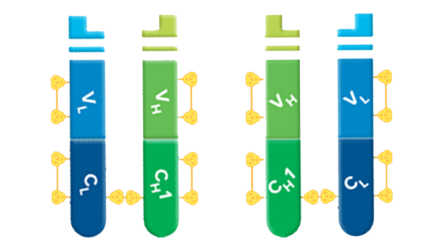
Fab fragments can be used to block endogenous immunoglobulins on cells, tissue, or other surfaces, and to block the exposed immunoglobulins in multiple labeling experiments using primary antibodies from the same species.
Anti-Mouse IgG Subclass Specific Antibodies
For distinguishing between two or more different subclasses of mouse IgG in multiple-labeling experiments, or for mouse IgG subclass determination.
Streptavidin
Conjugates of streptavidin are recommended for use with Biotin‑SP‑conjugated antibodies and Biotin‑SP‑conjugated ChromPure proteins.
Peroxidase-Anti-Peroxidase (PAP) Soluble Immune Complexes
PAP Soluble Immune Complexes are prepared by the method of Sternberger et al.
Solid Phase Immunoadsorbent Gels

For use in preparing affinity-purified antibodies or removing cross-reactive antibodies.
Signal Enhancement
IgG fraction Monoclonal Mouse Anti-Biotin, Anti-Fluorescein, Anti-Digoxin, and Affinity-purified anti-horseradish peroxidase for signal enhancement.
Normal Serums and Gamma Globulins

Normal serums are recommended as a blocking reagent to reduce background from non-specific, conserved sequence and/or Fc-receptor binding. Gamma Globulins are an inexpensive source of IgG.
Bovine Serum Albumin

Bovine serum albumin (BSA) is used extensively as a carrier protein to dilute antibodies and as a general protein blocking agent in immunoassays and immunodetection protocols.
Purified Proteins from Normal Serums
ChromPure is our trade name for highly purified proteins from the serum of non-immunized animals. Primarily for use as experimental controls.
Antisera to Immunoglobulins, Whole Serums, and Enzymes

Polyclonal antisera from immunized hosts are lipid extracted to improve clarity, salt fractionated, dialyzed against phosphate buffered saline containing sodium azide, and freeze-dried.
PolyHistidine Tags (His Tags) are a popular addition to recombinant proteins to facilitate protein purification and detection. His Tags are commonly used in preference to other tags due to the availability of inexpensive purification resins such as NiNTA. Anti-His Tag antibodies enable the detection of the tagged proteins allowing screening of expression. The poly-His Tag is usually formed of 6 histidine residues but may be between three and ten, usually at the C- or N-terminus of the recombinant protein. Here we demonstrate the utility of Jackson ImmunoResearch’s Anti-His Tag antibodies for the detection of His-Tagged proteins by both Western blotting and ELISA.
| Product Description | Product Code |
| AffiniPure Rabbit Anti-His Tag | 300-005-240 |
| Biotin-SP (long spacer) AffiniPure Rabbit Anti-His Tag | 300-065-240 |
| Alkaline Phosphatase AffiniPure Rabbit Anti-His Tag | 300-055-240 |
| Peroxidase AffiniPure Rabbit Anti-His Tag | 300-035-240 |
| Alexa Fluor® 488 AffiniPure Rabbit Anti-His Tag | 300-545-240 |
| Alexa Fluor® 790 AffiniPure Rabbit Anti-His Tag | 300-655-240 |
| Alexa Fluor® 680 AffiniPure Rabbit Anti-His Tag | 300-625-240 |
| Alexa Fluor® 647 AffiniPure Rabbit Anti-His Tag | 300-605-240 |
Confidently screen His-Tagged proteins in cell lysates by Western blot
Protein expression screening of His-Tagged recombinant proteins (constructs) from cell lysates is a popular application of Anti-His Tag antibodies. Anti-His Tag antibodies allow the detection of both carboxy- and amino- terminally His-Tagged proteins. Figure 1 demonstrates the detection of a recombinant His-Tagged protein from mammalian, insect, and bacterial cell lysates.
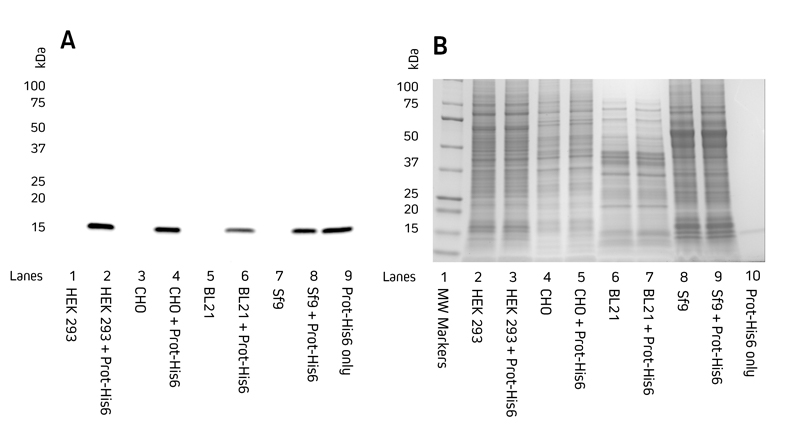
Figure 1. Detection of His-Tagged proteins in a range of cell lysates.
C-terminally His-Tagged protein was added at 100 ng to the cell lysates. Cell lysates of HEK 293, CHO, BL21 E. coli, and Sf9 insect cells were reduced and boiled for 5 minutes at 100°C and loaded at 9 μg/well in tandem SDS gels.
A. Western blot. Gels were electroblotted onto nitrocellulose membrane, blocked with 5% BSA in PBS/0.2% Tween 20, and probed with Jackson ImmunoResearch’s HRP Rabbit Anti-His Tag (300-035-240) at 1:20K dilution and visualized using a digital imager.
B. Gels were stained with Coomassie.
Use conjugated Anti-His Tag antibodies directly for single-step Western blotting
Jackson ImmunoResearch’s Anti-His Tag antibodies can be used directly using a Horseradish Peroxidase conjugate. Figures 2 and 3 demonstrate that Jackson ImmunoResearch’s HRP Rabbit Anti-His Tag antibody can be used across a range of dilutions and that sensitive detection of different His-Tagged proteins can be achieved. The sensitivity of the Western blot assay can be enhanced by performing the assay indirectly, using a conjugated secondary antibody to achieve increased signal shown in the following section.
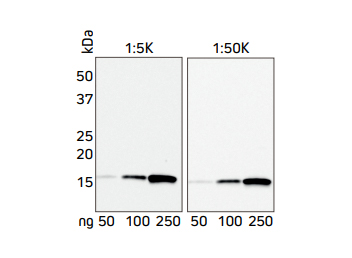
Figure 2. Western blot showing the detection of His-Tagged protein at two concentrations of HRP Rabbit Anti-His Tag antibody.
A recombinant His-Tagged protein was reduced and boiled for 5 minutes at 100°C and loaded in two sets of 50, 100 and 250 ng/well in an SDS gel. The gels were electroblotted onto a nitrocellulose membrane, which was then halved and each set of samples probed with Jackson ImmunoResearch’s HRP Rabbit Anti-His Tag (300-035-240) at either 1:5K or 1:50K. The resulting bands were visualized using a digital imager, the 1:5K blot was exposed for 3 seconds, and the 1:50K blot was exposed for 21 seconds.
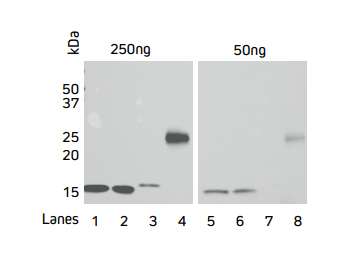
Figure 3. Direct Western blot showing the limit of detection for four recombinant His-Tagged proteins by HRP Rabbit Anti-His Tag antibody.
Four recombinant His-Tagged proteins were reduced and boiled for 5 minutes at 100°C and loaded into an SDS gel in two sets of 50 and 250 ng/well. Lanes 1, 2 and 4 have C-terminally tagged proteins while Lane 3 contains an N-terminally tagged protein. The gels were electroblotted onto a nitrocellulose membrane, and probed with Jackson ImmunoResearch’s HRP Rabbit Anti-His Tag (300-035-240) at a dilution of 1:20K. The resulting bands were visualized using a digital imager.
Enhance sensitivity by using Jackson ImmunoResearch’s Anti-His Tag antibody in combination with Anti-Rabbit conjugates
Signal enhancement can be achieved by indirect detection of the His Tag using an Anti-His Tag antibody followed by a conjugated Anti-Rabbit antibody such as an HRP Goat Anti-Rabbit secondary antibody. Figure 4 shows that sensitivity can be improved by using the indirect two-step method (B) in comparison to direct detection (A).
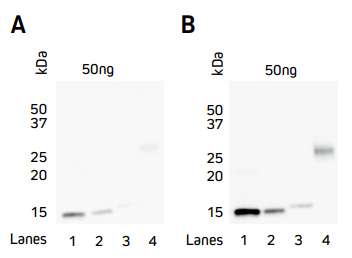
Figure 4. Comparison of direct and indirect Western blot detection of four recombinant His-Tagged proteins.
Samples
Lane 1: Protein 1 (C-term His Tag) – 50 ng
Lane 2: Protein 2 (C-term His Tag) – 50 ng
Lane 3: Protein 3 (N-term His Tag) – 50 ng
Lane 4: Protein 4 (C-term His Tag) – 50 ng
His-Tagged proteins were boiled and reduced in SDS-PAGE loading buffer containing 5% beta-mercaptoethanol (BME) and loaded at 50 ng/well as detailed above. Gels were electroblotted onto nitrocellulose and blots were probed. A) Direct: HRP Rabbit Anti-His Tag (300-035-240) at a dilution of 1:20K. B) Indirect: Rabbit Anti-His Tag (300-005-240) at a dilution of 1:5K followed by HRP AffiniPure Goat Anti-Rabbit IgG (H+L) (min X Hu, Ms, Rat Sr Prot) (111-035-144) at a dilution of 1:20K. Blots were visualized with a digital imager.
Anti-His Tag antibody for ELISA
Figure 5 shows the variation in binding characteristics of a range of recombinant His-Tagged Alpaca VHH antibodies isolated from an activity screen against antigen X and analyzed by ELISA. Variations in binding affinity of the different nanobodies can clearly be discerned and lead candidates selected.
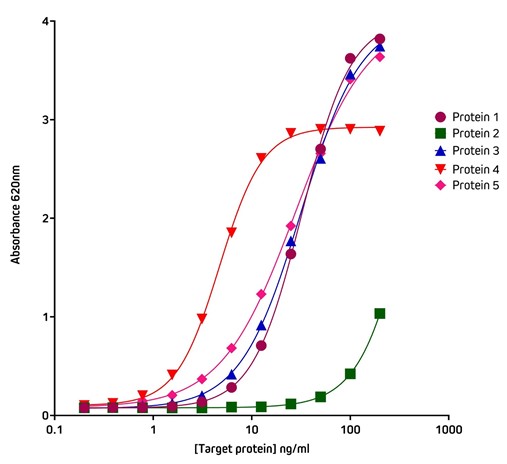
Figure 5. Detection of His-Tagged proteins with HRP Rabbit Anti-His Tag (300-035-240) by ELISA.
Coating Antigen: Antigen X was coated onto the plate at 1 μg/well (100 ul, 10 μg/ml). The plate was blocked with 1% Bovine Serum Albumin (IgG-Free, Protease-Free) (001-000-162) in PBST.
Sample: 5 individual purified His-Tagged recombinant Alpaca VHH Anti-Antigen X antibodies (Protein 1-5), loaded as serial dilutions of 20 ng (100 ul @ 200 ng/ml); 1:2 across the plate.
Detection Antibody: HRP Rabbit Anti-His Tag (300-035-240) diluted 1:20,000 (100 μl per well).
Plate incubated with TMB for 30 minutes at room temperature and read at 620 nm using a plate reader.
Screening ELISA using His Tag capture
The His-Tag offers another method of protein capture that may be useful when working with proteins subject to conformational sensitivity, allowing the protein to be elevated above the plate surface making more epitopes accessible to immunoreagents. Another application for the Anti-His Tag antibody is in situations where there is limited target protein-specific antibody availability, but the assay requires two different antibodies to generate the sandwich format. By capturing with the His Tag, target protein-specific antibodies can be used for detection in complex with conjugated species-specific secondary antibodies, allowing optimal signal amplification. His Tag capture also provides antibody-mediated, rapid and consistent binding of antigen to the ELISA plate.
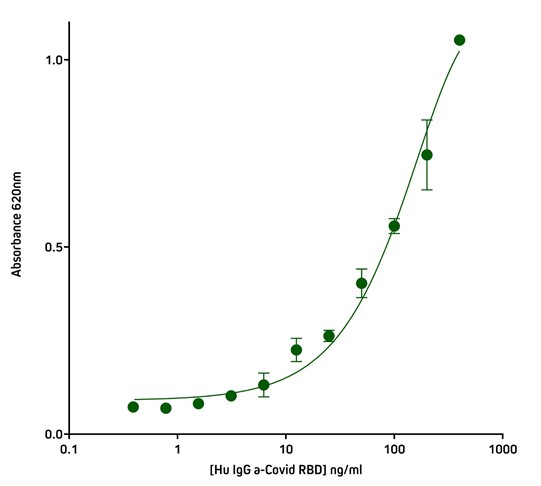
Figure 6. Detection of His-Tagged protein using Rabbit Anti-His Tag (300-005-240) by indirect Sandwich ELISA.
Capture Antibody: AffiniPure Rabbit Anti-His Tag (300-005-240) coated at 1 μg/well (100 μl, 10 μg/ml). The plate was blocked with 1% Bovine Serum Albumin (IgG-Free, Protease-Free) (001-000-162) in PBST
Sample: His-Tagged SARS-CoV-2 spike protein RBD (Genscript – Z03483-100) loaded at 20 ng/well (100 μl @ 200 ng/ml).
Detection Antibody 1: Human IgG anti-SARS-CoV-2 Spike S1 Antibody (Genscript A02038). 40 ng (100 ul @ 400 ng/ml) in first well; serially diluted 1:2 across plate.
Detection Antibody 2: HRP Goat Anti-Human IgG, Fcγ fragment specific (min X Bov, Ms, Rb Sr Prot) (109-035-170) diluted 1:20,000 (100 μl per well).
Plate incubated with TMB for 30 minutes at room temperature and read at 620 nm using a plate reader.
* We recommend using a detection antibody cross-adsorbed against rabbit serum proteins when using a rabbit antibody during the capture step to avoid background. Jackson ImmunoResearch antibodies, including anti-human, are available with minimal cross-reactivity to a variety of hosts, allowing you to abrogate background from multiple host species.
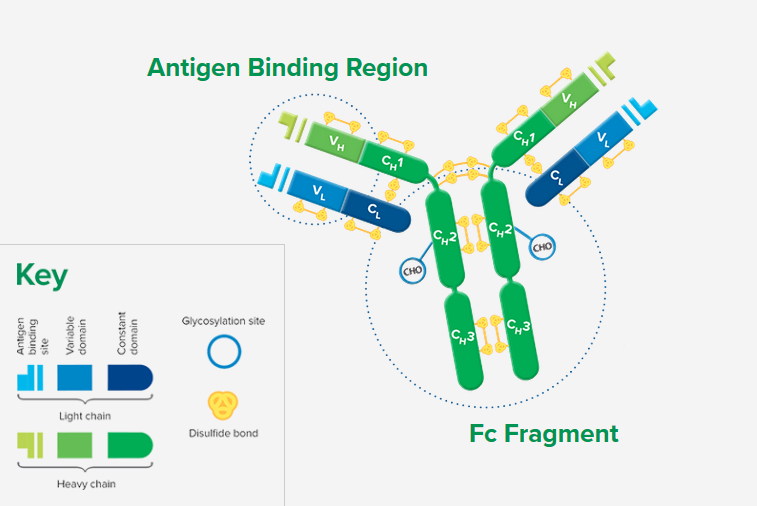
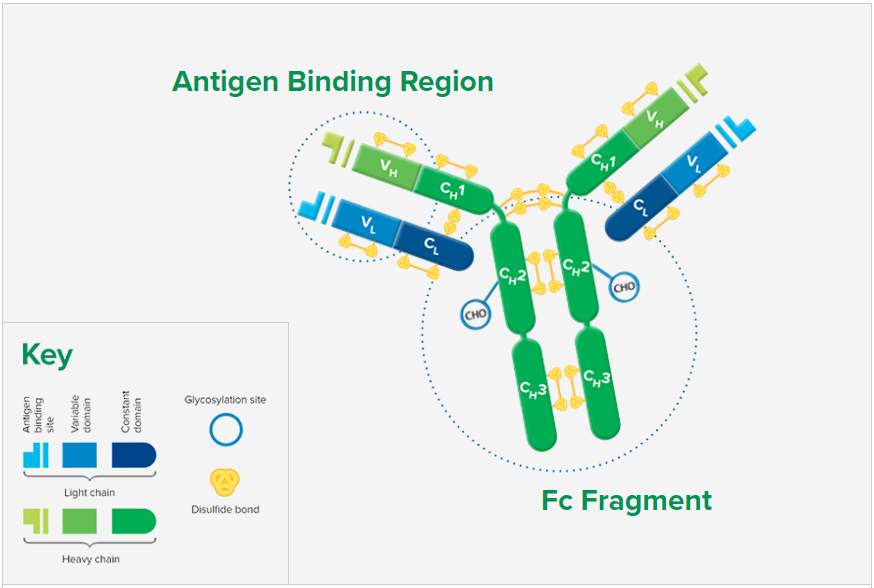
Whole IgG antibodies are isolated as intact molecules from antisera by affinity chromatography. They have an Fc portion and two antigen binding Fab portions joined together by disulfide bonds (Figure 1), and therefore are divalent. The average molecular weight is reported to be about 160 kD. The whole IgG form of antibodies is suitable for the majority of immunodetection procedures and is the most cost effective.
Whole IgG Secondary Antibodies For Specific Purposes
Note that these groups may include antibodies or conjugates not included in the main table of whole IgG secondary antibodies.
For flow cytometry we offer three fluorescent proteins (R-PE, APC, and PerCP) conjugated to many highly adsorbed whole IgG and F(ab’)2 fragment secondary antibodies. Also shown in this table are the same highly adsorbed antibodies conjugated to Biotin-SP and fluorescent dyes appropriate for flow cytometry (Alexa Fluor® 488, FITC, and Alexa Fluor® 647). Note that many antibodies listed elsewhere in tables of Whole IgG and F(ab’)2 fragments also can be used for flow cytometry.
Antibodies for Signal Enhancement
IgG fraction Monoclonal Mouse Anti-Biotin, Anti-Fluorescein, and Anti-Digoxin may be used either as direct conjugates, or for more sensitivity, they can be used unconjugated followed by a conjugated anti-mouse IgG (H+L) for signal enhancement.
Affinity-purified anti horseradish peroxidase (HRP) may be used to detect HRP or to enhance signal by binding to HRP-conjugated molecules.
A 50 kDa protein may be detected on blots without interference from the precipitated IgG in the same band, by incubation with its unlabeled primary antibody followed by labeled anti-IgG, Light Chain Specific secondary antibody.
Alexa Fluor® 680 and Alexa Fluor® 790 for High Sensitivity
Antibodies conjugated with far-red- and Infrared-emitting dyes are more sensitive than those with dyes emitting visible light due to low fluorescence quenching of the conjugates, high extinction coefficients of the dyes, and low background autofluorescence.
Cyanine dyes are better able to withstand the harsh dehydration and embedding conditions required for mounting sections in non-polar plastic media.
Anti-Mouse IgG Subclass Specific Antibodies
These antibodies have been extensively adsorbed against other mouse IgG subclasses. They are intended for distinguishing between two or more different subclasses of mouse IgG in multiple-labeling experiments, or for mouse IgG subclass determination.
Colloidal gold reagents are available either for transmission and scanning electron microscopy (EM Grade) or for light microscopy and immunoblotting (LM Grade).
Anti-Alpaca Secondary Antibodies
Jackson ImmunoResearch offers a range of secondary antibodies utilizing the properties of camelid immunoglobulins, for detection in assays and to expedite VHH development.
Anti-Human secondary antibodies are an invaluable reagent in the development of high-throughput serological testing kits.
Anti‑Chicken antibodies are useful as a component of Lateral flow immunoassays
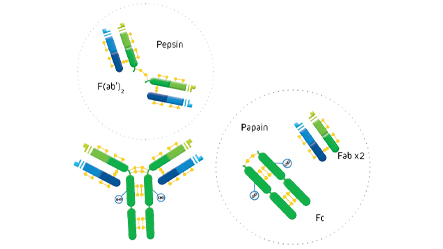
F(ab’)2 fragment antibodies are generated by pepsin digestion of whole IgG antibodies to remove most of the Fc fragment while leaving some of the hinge region intact. F(ab’)2 fragments have two antigen-binding (Fab) regions linked by disulfide bonds, making them divalent. The average molecular weight is approximately 110 kDa. They are used in specific applications, such as avoiding recognition of secondary antibodies by Fc receptors on live cells, or to Protein A or Protein G
Digestion of Whole IgG to Create F(ab’)2 Fragments
Binding of primary antibodies to Fc receptors also may occur if they are whole IgG antibodies, creating background regardless of the secondary antibody format. To block whole IgG primary and secondary antibodies from binding to Fc receptors, incubate cells in buffer containing 5% normal serum from the host species of the labeled secondary antibody. To prevent capping, endocytosis, and regeneration of Fc receptors on living cells, incubate at 4°C in buffer containing 5% normal serum with sodium azide added to inhibit metabolism.
Fab fragment antibodies are generated by papain digestion of whole IgG antibodies to remove the entire Fc fragment, including the hinge region. These antibodies are monovalent, containing only a single antigen binding site. The molecular weight a Fab fragment is about 50 kDa. They can be used to block endogenous immunoglobulins on cells, tissues or other surfaces, and to block the exposed immunoglobulins in multiple labeling experiments using primary antibodies from the same species.
Digestion of Whole IgG to Create Fab Fragments and an Fc Fragment
Fab Fragments Can Be Utilized in Three Key Ways:
Why Use Fab Fragments?
Monovalent Fab fragments of affinity-purified secondary antibodies are offered to block endogenous immunoglobulins in tissue sections or on cell surfaces, to cover (block) immunoglobulins when double labeling primary antibodies from the same host species, or to Fab-label primary antibodies prior to incubation with the experimental sample. They can be used for these purposes because each Fab fragment has only a single antigen binding site (i.e. they are monovalent), and they are non-precipitating.
Why is Monovalency Important?
Divalent (whole IgG or F(ab’)2 fragment) antibodies are not recommended for blocking because they have two antigen binding sites. After binding endogenous IgG or the first primary antibody, some antigen binding sites on a divalent secondary antibody may remain unoccupied, which could capture a primary antibody introduced in a subsequent step. Capture of the primary antibody would allow detection of that primary by a labeled secondary antibody, resulting in background staining or apparent signal overlap. The use of monovalent Fab fragments avoids this problem.
Selected literature references:
- Wessel and McClay, J. Histochem. Cytochem. 1986. 34, 703
- Franzusoff et al., J. Cell Biology. 1991. 112, 27
- Lewis Carl et al., J. Histochem. Cytochem. 1993. 41, 1273
- Negoescu et al., J. Histochem. Cytochem. 1994. 42, 433
Mouse IgG subclasses
Mice express 4 of the 5 available IgG subclasses making up their IgG isotype. They typically encode for IgG1, IgG2b and IgG3, and depending on their strain will also express either IgG2a or IgG2c (Collins 2016). IgG2a and IgG2c subclasses are seen as functionally comparable, being the most active of the subclasses to bind complement (Collins 2016).
Inbred mouse strains, such as C57Bl/6, C57Bl/10, SJL and NOD mice, possess an IgH-1b allele which results in expression of IgG2c instead of the IgG2a subclass expressed by BALB/c and Swiss Webster mice. The IgH-1a haplotype of these mice strains include the IGHG2C gene alongside the other isotypes, but not the IGHG2A present on the IgH-1b haplotype of the BALB/c mice (Martin et al 1998).
A number of monoclonal antibodies originate from these inbred stains, and some IgG2c clones have been incorrectly isotyped as IgG2a by reagents that cannot distinguish between these two subclasses. Subclass specific antibodies from Jackson ImmunoResearch provide exquisite discrimination among the subclasses.
Distinguish between different mouse IgG subclasses
Jackson ImmunoResearch anti-mouse IgG, subclass specific antibodies offer specificity to the 5 individual mouse IgG subclasses. These highly specific antibodies are designed to distinguish between two or more different subclass of mouse IgG in multiple labeling experiments, or for mouse IgG subclass determination.
They have been adsorbed against human, bovine and rabbit serum proteins to minimize interference from cross-reactivity with tissue immunoglobulins, adherent bovine IgG on cultured cells and rabbit primary antibodies.
Anti-Mouse IgG, subclass specific antibodies are available conjugated to Alexa Fluor® and Cyanine™ Fluorescent dyes, Biotin-SP™, and horseradish peroxidase and alkaline phosphatase reporter molecules.
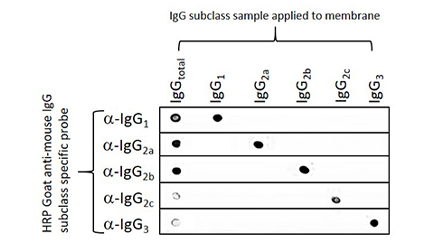
The example below illustrates the performance of the Goat Anti-Mouse subclass specific antibodies in a dot blot experiment. In each case, the subclass specific antibody only recognizes its specific target in the presence of additionally blotted subclasses.

Figure 2: Dot blot showing the specificity of goat anti-mouse IgG, Fcγ subclass specific antibodies. Separate nitrocellulose strips (rows) received 100 ng “dots” of mouse IgG and each subclass, and then were blocked with 5% (w/v) BSA in PBST. After probing with Peroxidase-conjugated goat anti-mouse subclass specific antibodies, the strips were developed with TMBM substrate from Moss, Inc. The grid of positive signals shows the specificity of each subclass directed antibody. Some subclasses are poorly represented in a total IgG pool (IgGtotal, α-IgGs 2c and 3) and thus give weak signal. Peroxidase conjugates used for probing were 115-035-205 (anti-mouse IgG1), 115-035-206 (anti-mouse IgG2a), 115-035-207 (anti-mouse IgG2b), 115-035-208 (anti-mouse IgG2c), and 115-035-209 (anti-mouse IgG3).
| *Subclass specific antibodies are not necessary for general detection of mouse monoclonal antibodies in single-labeling experiments or in multiple-labeling experiments involving one mouse monoclonal and primary antibodies from other species. |
References:
Collins, A. (2016). IgG subclass co-expression brings harmony to the quartet model of murine IgG function. Immunol Cell Biology, 94, 10, 949- 954. http://dx.doi.org/10.1038/icb.2016.65
Martin, R., Brady, J., Lew, A. (1998) The need for IgG2c specific antiserum when isotyping antibodies from C57BL/6 and NOD mice, Journal of Immunological Methods, Volume 212, Issue 2, 1998, Pages 187-192, ISSN 0022-1759. http://dx.doi.org/10.1016/S0022-1759(98)00015-5
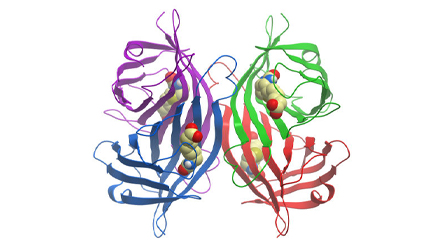
Streptavidin is a tetrameric bacterial protein isolated from Streptomyces avidinii providing 4 high-affinity biotin binding sites (Figure 1). Comparisons of apo and liganded streptavidin crystal structures by Weber et al. (1989) showed that affinity is conferred by multiple hydrogen bonds and van der Waals interactions, which in conjunction with polypeptide loops confine the biotin in streptavidin’s interior. The result is one of the strongest non-covalent bonds found in nature, with a femtomolar dissociation constant (Kd ~10-15). Unlike egg-white avidin, which has a net positive charge at neutral pH and contains about 7% carbohydrate, streptavidin has almost no net charge at neutral pH, does not contain carbohydrate, and exhibits lower non-specific background.
Because of its binding characteristics, streptavidin is commonly employed for immunotechniques requiring signal amplification using biotinylated reagents.
Jackson ImmunoResearch streptavidin conjugates are recommended for use with Biotin-SP-conjugated affinity-purified secondary antibodies and ChromPure™ proteins, as well as with any biotinylated primary or secondary antibody, or oligonucleotide.

Signal Amplification with Streptavidin
A number of signal amplification techniques are possible with Jackson ImmunoResearch secondary antibodies. Streptavidin is offered for signal enhancement (Figure 2) as a superior technique to the avidin-biotin-HRP complex (ABC) method. Compared with the ABC method, HRP-conjugated streptavidin is more stable, gives less background, and is more sensitive as reported by Shi et al. (1988) and Milde et al. (1989). The increased sensitivity may be due to enhanced tissue penetration and less steric hindrance, since nominal molecular weights for all components of HRP conjugated Streptavidin total less than 200 kDa, considerably lower than the weight of ABC.
Jackson ImmunoResearch offers a comprehensive list of fluorophores and enzymes conjugated to streptavidin for use in enzyme immunoassays, immunohistochemistry, flow cytometry, in situ hybridization, and immunoblotting procedures. Most streptavidin products are freeze-dried in buffer containing stabilizers and preservative. Exceptions are unconjugated streptavidin (freeze-dried from a sodium chloride solution), HRP-conjugated streptavidin (freeze-dried without preservative) and alkaline phosphatase-conjugated streptavidin (sterile-filtered liquid with preservative).
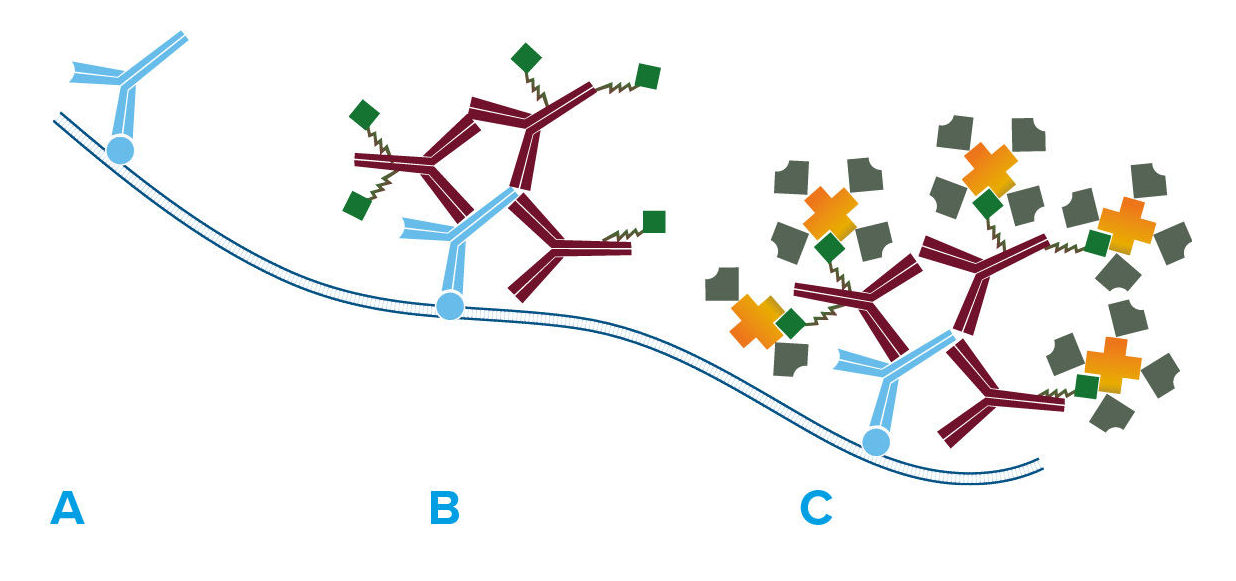
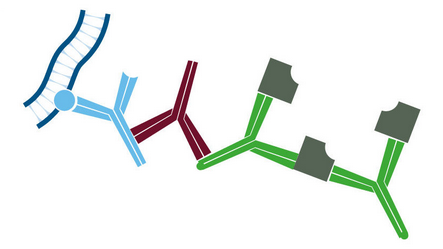
Figure 2. Labeled streptavidin biotin method (LSAB).
- Primary antibody detects target antigen.
- Biotinylated secondary antibody recognizes primary antibody.
- HRP-conjugated streptavidin binds biotinylated secondary antibody, enhancing the signal compared with HRP-conjugated secondary antibody.
References
- Weber, PC., Ohlendorf, DH., Wendoloski, JJ., Salemme, FR. (1989) Structural origins of high-affinity biotin binding to streptavidin. Science., 1989 Jan 6;243(4887):85-8
- Shi, ZR., Itzkowitz, SH., Kim, YS., (1988) A comparison of three immunoperoxidase techniques for antigen detection in colorectal carcinoma tissues. Journal of Histochemistry & Cytochemistry Vol 36, Issue 3, pp. 317 – 322
- Milde, P., Merke, J., Ritz, E., Haussler, MR., Rauterberg, EW., (1989) Immunohistochemical detection of 1,25-dihydroxyvitamin D3 receptors and estrogen receptors by monoclonal antibodies: comparison of four immunoperoxidase methods. Journal of Histochemistry & Cytochemistry Vol 37, Issue 11, pp. 1609 – 1617
PAP soluble immune complexes are prepared by the method of Sternberger et al. (J Histochem. Cytochem. 1970. 12, 315). Theoretically, they consist predominantly of two anti-HRP antibodies in soluble complex with three molecules of HRP.
PAP soluble complexes are normally used at 25-50 μg/ml. Whole antisera against IgG (H+L) are recommended for bridging PAP to primary antibodies. Antisera against IgG (H+L) or against the F(ab’)2 fragments of IgG will also bridge PAP to IgM primary antibodies (such as IgM monoclonal antibodies) by virtue of common light-chain recognition. Normal serum (5% v/v) from the same host species as the bridging antibody is suggested as a blocking solution to minimize non-specific binding. All PAP soluble complexes are freeze-dried at 20 mg/ml.
Endogenous peroxidase activity: PAP soluble complexes, as well as other immunoperoxidase reagents, are not recommended for tissues or cells in which endogenous peroxidase activity is difficult to suppress. In such cases, other immunoenzyme reagents may be used. Alternatively, anti-horseradish peroxidase conjugated with other enzymes or fluorophores may be used to enhance signal and reduce background, since the final signal does not depend on the enzyme activity of peroxidase, but on the antigenicity of horseradish peroxidase.

A. Primary antibody is bound to antigen, and a bridging antibody has been added to link the primary antibody with corresponding PAP complex.
B. PAP complex (three molecules of HRP complexed with two molecules of anti-HRP) has been captured by bridging antibody.
Highly purified IgG from the serum of non-immunized animals is coupled to cyanogen bromide-activated 4% agarose gels for use in preparing affinity-purified antibodies or removing cross-reactive antibodies. Proteins are coupled at a concentration of 1 mg protein per ml of settled gel, and are packaged in phosphate buffered saline with sodium azide.
Anti-Biotin, Anti-Fluorescein and Anti-Digoxin antibodies
Monoclonal Mouse Anti-Biotin, Anti-Fluorescein and Anti-Digoxin are available in a wide range of conjugate options for direct detection of their target molecules, which are commonly used as tags on proteins and nucleic acids. If signal amplification is desired, these antibodies can be used unconjugated, followed by conjugated anti-mouse IgG (H+L) (see examples below using Mouse Anti-Biotin).
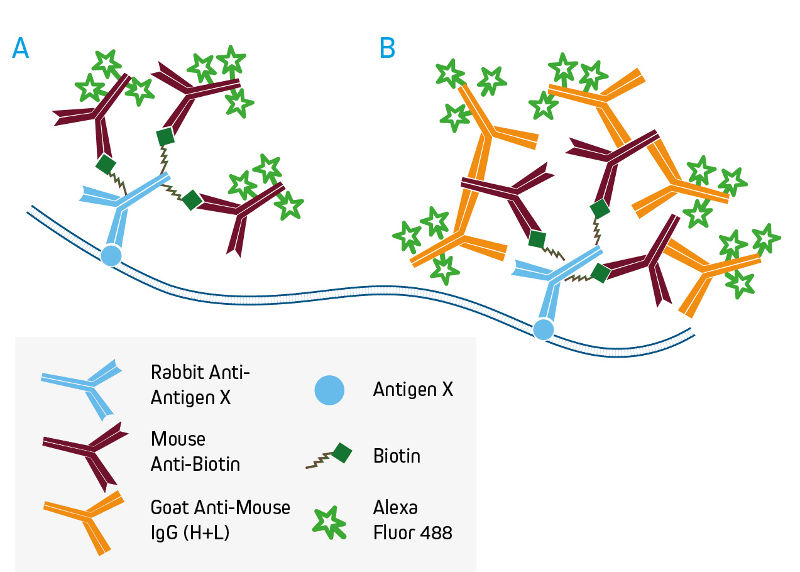
A. Direct detection of biotinylated primary antibodies with conjugated Mouse Anti-Biotin.
B. Signal enhancement with conjugated Anti-Mouse IgG (H+L).
Detection with Anti-HRP antibodies
Affinity-purified anti-horseradish peroxidase (HRP) may be used to detect HRP or to enhance signal by binding to HRP-conjugated molecules. Anti-HRP also may be used to convert an HRP conjugate into a different signal as illustrated in the example below using ImmunoGold-complexed anti-HRP. Goat Anti-HRP is also available conjugated to alkaline phosphatase and fluorescent probes, providing additional flexibility to the detection method.
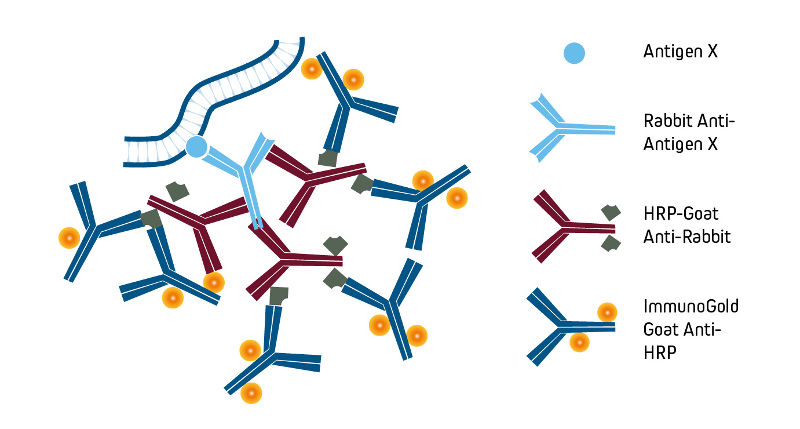
Preparation and format
Normal serums (from non-immunized animals) are lipid extracted to improve clarity, dialyzed against phosphate buffered saline (PBS) containing sodium azide, and freeze-dried.
Applications
Normal serum diluted to 5% (v/v) in PBS is strongly recommended as a blocking reagent to reduce background from non-specific, conserved-sequence and/or Fc-receptor binding. Best results are obtained with diluted normal serum from the same host as the labeled antibody, as a separate incubation step before addition of the primary antibody.
Gamma Globulins
Gamma globulins are further purified from non-immunized animal serums by salt fractionation, ion-exchange chromatography, and gel filtration. Gamma globulins are an inexpensive source of IgG with only trace amounts of other immunoglobulins and/or non-immunoglobulin serum proteins. Gamma globulins are supplied as sterile liquids in phosphate buffer without stabilizers or preservatives.
Applications
Bovine Serum Albumin (BSA) is used extensively as a carrier protein to dilute antibodies and as a general protein blocking agent in immunoassays.
JIR Bovine Serum Albumin is verified to be IgG- and protease-free, alleviating many problems associated with commonly available preparations.

Note: Most commercial preparations of BSA, including some of the highest purity grades, contain contaminating bovine IgG that may become an antigen for cross-reacting secondary antibodies. This is particularly common when using anti-bovine IgG, anti-goat IgG (with the exception of bovine anti-goat IgG), anti-horse IgG, or anti-sheep IgG, but may occur with other antibodies that cross-react with bovine IgG. The result of these interactions may be loss of desired antibody activity, loss of antibody stability, and/or increased background.
Secondary antibody activity may be lost if the antibody is diluted in BSA that contains contaminating bovine IgG. Background may derive from sticky soluble immune complexes in the antibody diluent, or from bovine IgG found in a BSA blocking solution which becomes a target for labeled secondary antibodies. Even small amounts of contaminating IgG may create these problems, due to high concentrations of BSA in many protocols.
Format
IgG-free BSA is supplied as a pure protein, freeze-dried from deionized water. Please inquire about availability of larger sizes.
ChromPure™ Proteins (from Normal Serums) and Conjugates
ChromPure is our trade name for highly purified proteins from the serum of non-immunized animals. The purified immunoglobulins in this section do not represent antibodies directed against known antigens.
Preparation
ChromPure proteins are prepared by a variety of methods, including ion-exchange, gel-filtration, hydrophobic, dye-ligand, metal-affinity, Protein A, and immunoaffinity chromatographies. Enzyme digestion is used to generate F(ab’)2 (pepsin), and Fab and Fc (papain) fragments from highly purified whole molecules.
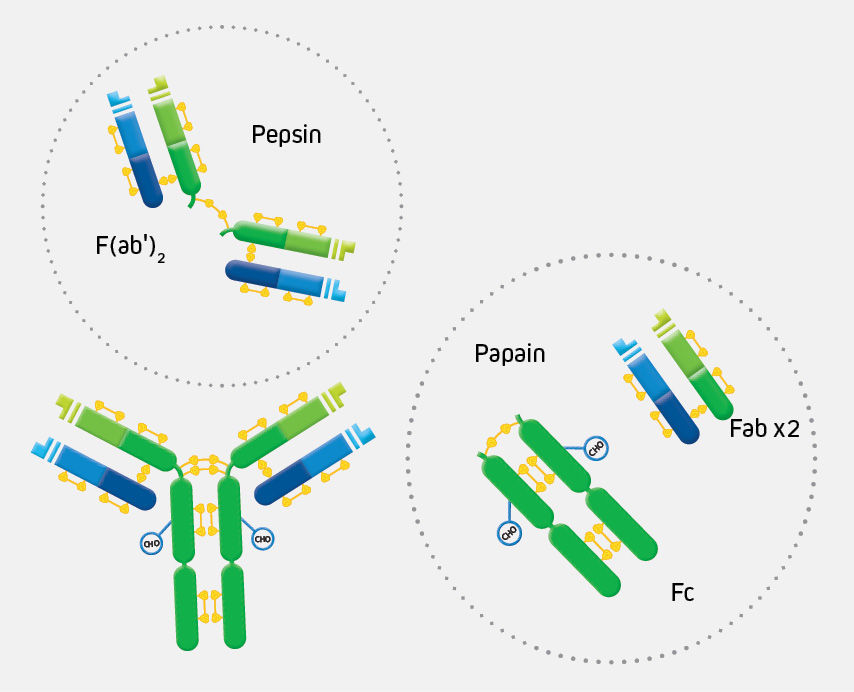
The digestion of IgG (approximate MW = 160 kDa) yields a number of different fragments. Papain digestion generates two monovalent Fab fragments and an Fc fragment, each about 50 kDa. Pepsin digestion degrades the Fc domain, leaving a divalent F(ab’)2 fragment with approximate MW of 110 kDa.
Purity
No contaminating whole molecules or undesired fragments are observed at a protein concentration of 20 mg/ml when tested by immunoelectrophoresis against anti-whole serums, anti-immunoglobulins (class specific), or anti-fragment specific antisera.
Applications
ChromPure proteins are ideal for use as experimental controls (isotype controls).
Note: ChromPure proteins are not intended for use as immunogens to produce monospecific antibodies, nor should they be used as molecular weight markers.
Format
Unconjugated ChromPure proteins are supplied as sterile-filtered liquids without stabilizers or preservative. Conjugated ChromPure proteins are freeze-dried with stabilizers and a preservative, with the exception of peroxidase conjugates, which do not contain a preservative.
Polyclonal antisera from immunized hosts are lipid extracted to improve clarity, salt fractionated, dialyzed against phosphate buffered saline containing sodium azide, and freeze-dried. Antisera against whole serums are obtained by immunizing host animals with whole serum. Antisera against whole IgG molecules [i.e. Anti-IgG (H+L)] are recommended for bridging PAP to primary antibodies.