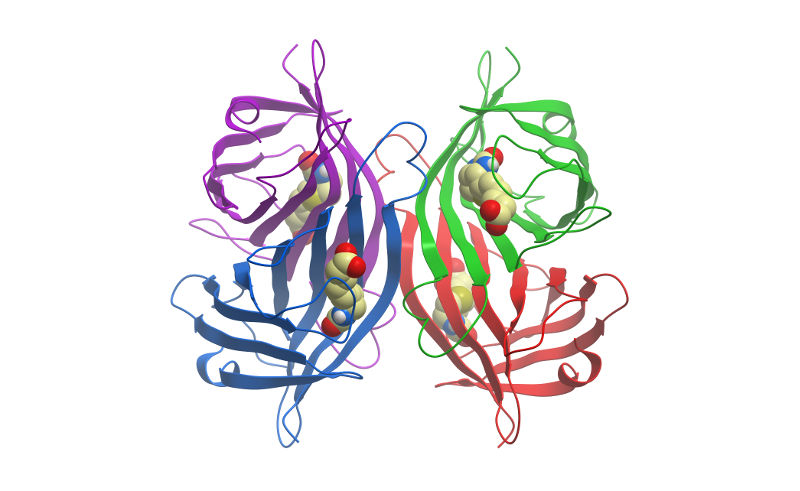
Streptavidin is a tetrameric bacterial protein isolated from Streptomyces avidinii providing 4 high-affinity biotin binding sites (Figure 1). Comparisons of apo and liganded streptavidin crystal structures by Weber et al. (1989) showed that affinity is conferred by multiple hydrogen bonds and van der Waals interactions, which in conjunction with polypeptide loops confine the biotin in streptavidin’s interior. The result is one of the strongest non-covalent bonds found in nature, with a femtomolar dissociation constant (Kd ~10-15). Unlike egg-white avidin, which has a net positive charge at neutral pH and contains about 7% carbohydrate, streptavidin has almost no net charge at neutral pH, does not contain carbohydrate, and exhibits lower non-specific background.
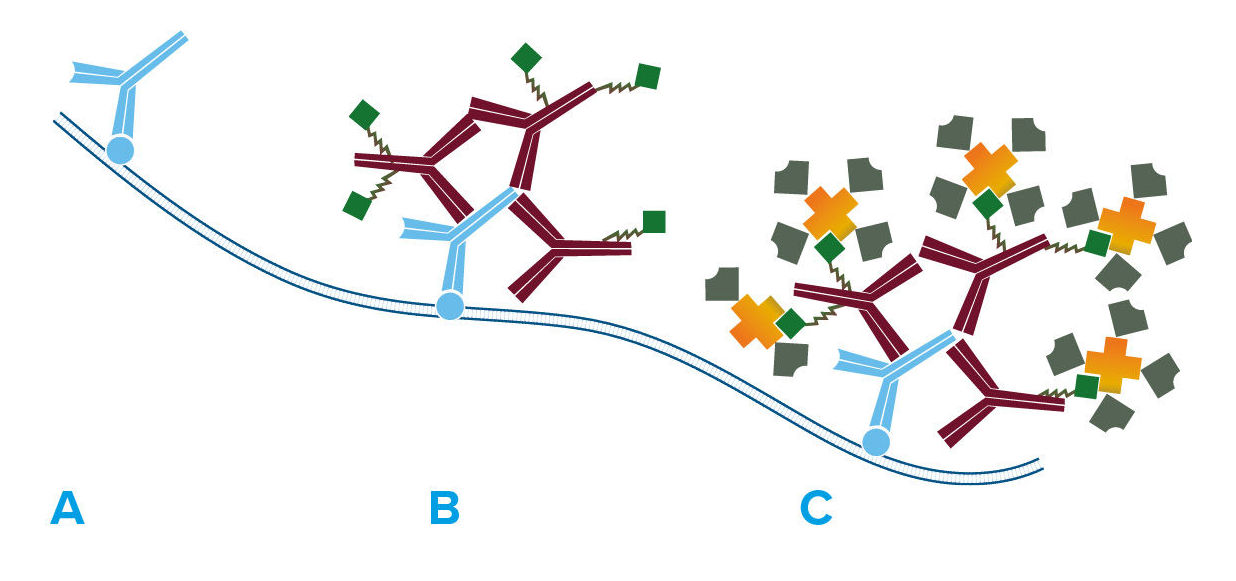
Because of its binding characteristics, streptavidin is commonly employed for immunotechniques requiring signal amplification using biotinylated reagents.
Jackson ImmunoResearch streptavidin conjugates are recommended for use with Biotin-SP-conjugated affinity-purified secondary antibodies and ChromPure™ proteins, as well as with any biotinylated primary or secondary antibody, or oligonucleotide.