Gene Editing Reagents
Products
Overview
Gene therapy generally functions by introducing exogenous genes (or gene-editing tools) into target cells or tissues, serving to replace, compensate, block, correct, augment, or knockout specific genes to exert therapeutic effects [1]. Based on the different methods of introducing genes into the human body, gene therapy can be divided into ex vivo therapies (extracorporeal therapies) and in vivo therapies (intracorporeal therapies). Ex vivo therapy generally involves introducing exogenous genes (or gene-editing tools) into cells outside the body, preparing them to become genetically modified cells or cell-derived products, and ultimately reintroducing them to the body to exert therapeutic effects. In vivo therapy introduces exogenous genes (or gene-editing tools) into the human body via non-viral vectors (such as LNP) or viral vectors (such as Adeno-Associated Virus AAV, Lentivirus LV, etc.) to exert therapeutic effects.
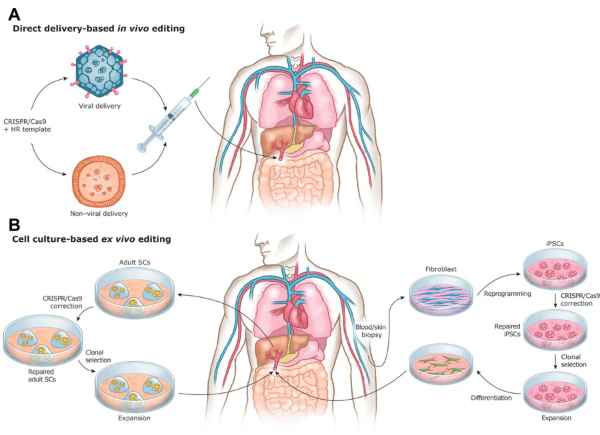
Figure 1. Types of gene editing [1][2].
GMP Cas9 & Cas12a Nucleases
Cas9 and Cas12a are primarily used for the genetic modification of cells and gene therapy drugs, including hematopoietic stem cells and T cells. Our GMP-Grade Cas9 nuclease is manufactured in a high-standard GMP-Grade enzyme production facility and has undergone rigorous in vitro and intracellular quality validation. KACTUS has filed our GMP Cas9 with the FDA Drug Master Files (DMF# 036578). KACTUS has provided regulatory support to customers completing Investigational New Drug (IND) applications with our Cas9.
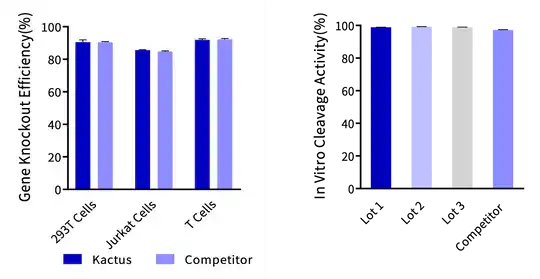
Figure 2. (Left) Cas9 Nuclease is used for gene knockout in 293T cells, Jurkat cells, and T cells, and its gene knockout efficiency in various cell types is comparable to that of leading suppliers. (Right) Cas9 Nuclease In Vitro DNA Cleavage Experiment. The in vitro cleavage efficiency of Cas9 nuclease is equivalent across all three batches.
Cas9 ELISA Kit
KACTUS Cas9 ELISA kit is for quantitative detection of residual Cas9 nuclease in gene-edited cell drugs before reinfusion into the human body, whether extracellular or intracellular. Our test kit has a detection range of 0.25 ng/mL to 16 ng/mL, with sensitivity reaching up to 0.125 ng/mL.
High Accuracy and Standard Curve R² > 0.99
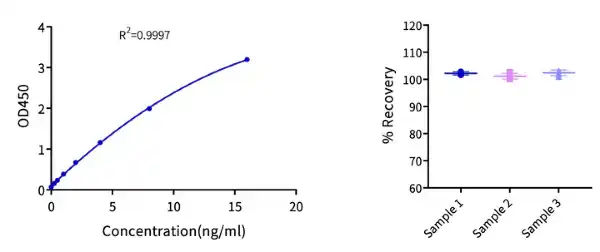
Figure 3. (Left) Cas9 ELISA Kit example standard curve with an R² of 0.9997. (Right) The same test kit was used to assess the spike-recovery rate of Cas9 nuclease at three different concentrations (high, medium, low) in dilution buffer. The spike-recovery rate for different concentrations of Cas9 samples in dilution buffer all fell between 80%-120%, indicating that this ELISA kit has high accuracy in detecting residual Cas9 nuclease.
GMP-Grade DNA/RNA Degradation with MaxNuclease
In gene therapy, MaxNuclease principally serves to eliminate contaminants like host DNA and plasmid DNA present during the pharmaceutical production process. This GMP-grade nuclease is produced within a facility maintaining stringent GMP-Grade production standards, avoiding the use of materials derived from animals. The analytical methods applied have undergone systematic validation, and the final enzyme is subject to extensive quality control release testing to ensure it meets the requirements for use from research and development through to large-scale commercial production. Additionally, KACTUS has submitted MaxNuclease to the FDA Drug Master Files (DMF #036799).
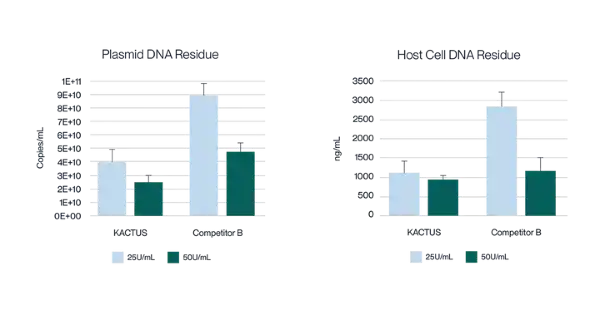
Figure 4. Virus harvest solution was treated with 25U/mL and 50U/mL endonuclease at 37°C for 2 hours, respectively. Detection of plasmid DNA (pDNA) residue (left) and host cell DNA (HCD) (right) residue was analyzed. KACTUS MaxNuclease has higher degradation activity versus Competitor B demonstrated by lower pDNA and HCD residue for both 25U/mL and 50U/mL working concentrations.
MaxNuclease ELISA Kit
The quantification of residual MaxNuclease in gene therapy drugs is achievable with our associated ELISA kit, which provides a wide detection range of 46.88pg/mL to 3000pg/mL and sensitivity reaching up to 23.44pg/mL.
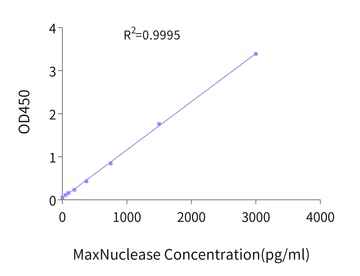
Figure 5. Example standard curve for MaxNuclease ELISA kit.
ELISA Kits for Residual AAV Quantification
AAV is a commonly used viral vector in the field of gene therapy. Methods for detecting the titer of AAV viral particles include Dot blot, TEM, AUC, ELISA, SEC-MALS, etc. KACTUS has developed an AAV5 and AAV9 Titration ELISA Kit, aiming to provide a more convenient and reliable solution for the quantification of residual AAV viral particle titer.
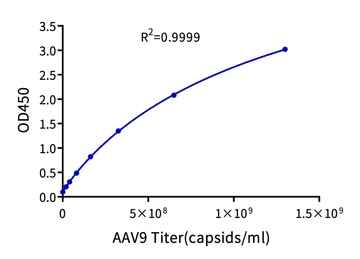
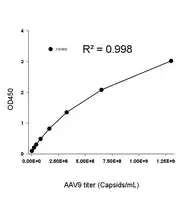
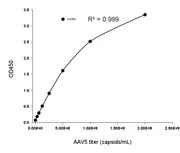
Figure 6. Example standard curve for the AAV9 Titration ELISA Kit, with a quantification range of 2.03E+07-1.30E+09 capsids/mL.
Specificity
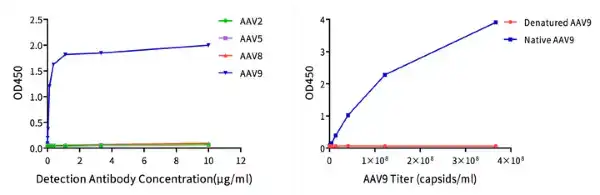
Figure 7. (Left) An indirect ELISA was used to detect the cross-binding of different concentrations of AAV9 antibodies with AAV2/5/8/9. A curve with antibody concentration as the x-axis and OD450 value as the y-axis is given. Results show that AAV9 detection antibodies only specifically bind to AAV9 and do not cross-react with the capsid proteins of AAV2/5/8.
(Right) A sandwich ELISA was used to detect the binding of AAV9 antibodies with both intact and denatured AAV9. A curve with AAV9 capsid titers as the x-axis and OD450 value as the y-axis is given. Results show that AAV9 detection antibodies do not bind to the denatured AAV9 capsid.
Accuracy
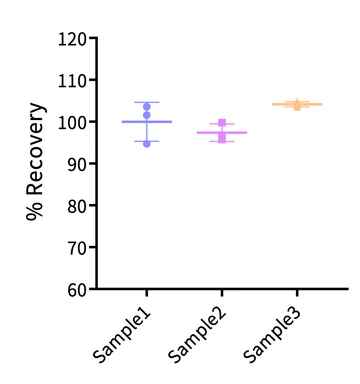
Figure 8. The same AAV9 kit was used to test the spiked recovery rate of high, medium, and low titer AAV9 samples in dilution buffer. The spike recovery rates of different titer AAV9 samples tested in dilution buffer are all between 80% and 120%, indicating that the kit has high accuracy in detecting AAV9 titers.
In Vitro Enzymatic Synthesis of DNA Vectors
The enzymatic synthesis of DNA vectors in vitro can effectively replace traditional plasmid DNA production, perfectly avoiding the many uncontrollable risks of the biological fermentation process, and can complete industrial production in a shorter time, achieving the goal of accelerating production and reducing costs. In the field of gene therapy, it is mainly used to prepare viral vectors such as AAV (Adeno-Associated Virus), LV (Lentivirus), etc.
TelN Protelomerase
TelN Protelomerase can be used to synthesize linear, closed mini DNA vectors. It cuts double-stranded DNA at the recognition site TelRL (56bp) and leaves a covalently closed hairpin structure at the enzyme cutting site, effectively converting circular DNA into linear DNA with hairpin ends. In practical applications, the target fragment and the backbone sequence on the plasmid are separated by TelRL sites.
Note: In some application scenarios, the use of this enzyme may be subject to patent restrictions.
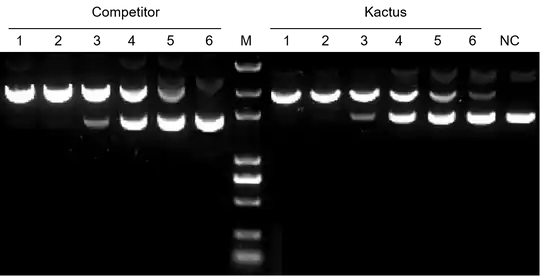
Figure 9. TelN Protelomerase can effectively convert circular DNA into linear DNA with hairpin ends. The activity of KACTUS TelN Protelomerase is comparable leading suppliers. In a 50 μL system, 323 fmol of dsDNA was added to 1 μL of TelN Protelomerase (wells 1-6 diluted in a 2-fold gradient respectively, with NC as the control group) in a 30 minute reaction at 37℃.
Phi29 DNA Polymerase
Phi29 DNA polymerase has strong strand displacement activity and characteristics of continuous synthesis, and can be used for synthesizing double-stranded DNA in vitro.
Note: The use of this enzyme may be subject to patent restrictions in some application scenarios.
ExoNuclease III & T5 Exonuclease
The DNA vectors formed by the cutting and linking of TelN Protelomerase are of two types: one contains the backbone sequence, and the other contains the target fragment. The backbone sequence has corresponding restriction enzyme cutting sites, forming blunt or sticky ends after enzymatic cutting. Further action by Exonuclease III or T5 Exonuclease achieves the degradation of the backbone sequence, serving the purpose of enriching the target fragment.

Figure 10. Exonuclease III & T5 Exonuclease effectively degrade double-stranded DNA linearized by BsaI (forming 5’ overhang ends after BsaI digestion), but have no effect on double-stranded DNA linearized by TelN Protelomerase (TelN Protelomerase linearization forms linear DNA with hairpin ends). In a 20 μl reaction system, add 2 μg of double-stranded DNA treated with BsaI/TelN Protelomerase and 1 μl of Exonuclease III/2 μl of T5 Exonuclease, and react at 37℃ for 30 min.
Note: In the figure, Exo III represents Exonuclease III; T5 Exo represents T5 Exonuclease.
Available Products
| Product Category | Catalog # | Product Name | Available Sizes |
| CRISPR Enzymes | GMP-CAS-EE109 | Cas9 Nuclease (GMP-Grade) | 3 mg |
| CRISPR Enzymes | CAS-MM00B | Cas9 ELISA Kit | 96 T |
| CRISPR Enzymes | CAS-EE121 | AsCas12a Nuclease | 1 mg |
| Nonspecific Endonuclease | GMP-NUC-SE101 | MaxNuclease (GMP-Grade) | 250kU / 5MU |
| Nonspecific Endonuclease | NUC-SE00B | MaxNuclease ELISA Kit | 96 T |
| AAV ELISA Kit | AV5-MM00B | AAV5 Titration ELISA Kit | 96 T |
| AAV ELISA Kit | AV9-MM00B | AAV9 Titration ELISA Kit | 96 T |
| DNA Synthesis | TLN-BE001 | TelN Protelomerase | 10kU |
| DNA Synthesis | PHI-BE101 | phi29 DNA Polymerase | 500U / 5000U |
| DNA Synthesis | EXO-EE101 | Exonuclease III | 5000U / 50kU |
Browse Gene Editing Reagent Products by Category
Dependable Quantification of AAV Capsid Titer
AAV Titration ELISA Kits with high specificity, sensitivity, and reproducibility
Reproducible quantification of AAV capsid titer using sandwich ELISA
Our kits use a double-antibody sandwich enzyme-linked immunosorbent assay (ELISA) to determine the quantity of AAV capsids in the test sample. The AAV monoclonal antibody is pre-coated on a 96-well reaction plate. AAV standard or sample is added to the pre-coated plate, which will specifically bind to the reaction plate. The biotinylated detection antibody is then added to bind the AAV capsids. Next, the HRP conjugate is added to form an antibody-antigen complex. HRP will then oxidize TMB substrate to produce a color change. The intensity of the color change is proportional to the AAV capsid titer in the sample. After reading the absorbance, the AAV capsid titer is calculated based on the absorbance values and the known AAV titers of the standard.
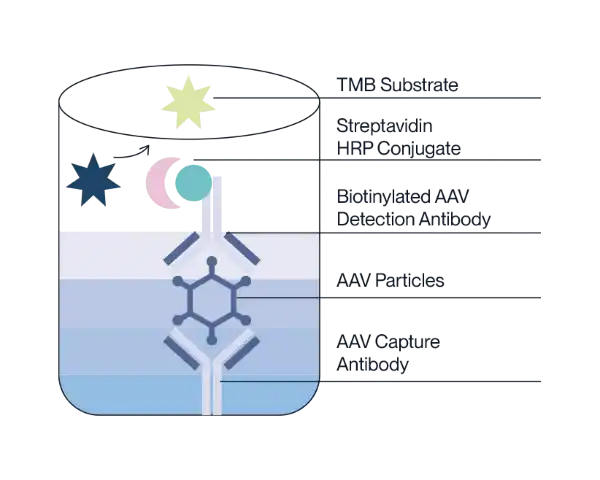
Available Products
| AAV2 Titration ELISA Kit | AAV5 Titration ELISA Kit | AAV6 Titration ELISA Kit | AAV8 Titration ELISA Kit | AAV9 Titration ELISA Kit | |
| Catalog Number | AV2-MM00B | AV5-MM00B | AV6-MM00B | AV8-MM00B | AV9-MM00B |
| Detection Range | 9.38E+07 capsids/mL – 6.00E+09 capsids/mL | 3.13E+07 capsids/mL – 2E+09 capsids/mL | 1.00E+08 capsids/mL – 6.40E+09 capsids/mL capsids/mL | 2.80E+07 capsids/mL – 1.79E+09 capsids/mL | 2.03E+07 capsids/mL – 1.30E+09 capsids/mL |
| Sensitivity | 5E+07 capsids/mL | 1.56E+07 capsids/mL | 1.40E+07 capsids/mL | 1.40E+07 capsids/mL | 1.02E+07 capsids/mL |
Advantages of KACTUS AAV ELISA Kits

High sensitivity & precision
Recombinant antibodies for reproducibility
Low background
Specificity to individual AAV serotype
Wide linear range
Why use ELISA kits for quantification of AAV particles?
Empty AAV capsids induce immune response without therapeutic benefit
Empty AAV capsids lacking the transgene of interest cause an unwanted immune response without providing the therapeutic benefit of the transgene. Alongside DNA and infectious titers, determination of the capsid titer is crucial for validating the efficacy and safety of your AAV gene therapy. Our titration kits detect full and empty AAV capsids.
As the gene therapy market using adeno-associated virus (AAV) as a vector continues to rapidly develop, regulatory agencies have imposed increasingly stringent requirements for quality control (QC) testing of AAV drugs. To ensure the safety and efficacy of AAV drugs, there is a need for effective, reliable, and stable methods to quantify AAV viral particle titers.
ELISA Demonstrates High Specificity and Reproducibility for AAV Capsid Titer
Currently, there are several methods for AAV viral particle titer quantification, including UV spectrophotometry, enzyme-linked immunosorbent assay (ELISA), and high-performance liquid chromatography (HPLC), among others. Each method has its own advantages and limitations. Among them, ELISA has gained widespread application due to its high reproducibility and good specificity, making it one of the recommended methods for titer determination by regulatory agencies.
KACTUS has developed an AAV5 and AAV9 ELISA kit for quantifying AAV viral particle titers of different serotypes. These test kits provide accurate, reproducible, stable, and reliable results for the quantitative analysis of AAV viral particles.
Example Standard Curve
Our AAV ELISA kits use an 8-point standard curve of recombinant AAV capsids. Monoclonal antibodies specific to the AAV serotype are precoated on the plate and bind the AAV capsids. Additional biotinylated AAV monoclonal antibodies bind as the second label
Catalog #GMP-NUC-SE101
Degrades all DNA & RNA for biologics manufacturing
About MaxNuclease™
What is MaxNuclease?
Non-Specific Endonuclease from Serratia marcescens
MaxNuclease is identified from Serratia marcescens and is genetically engineered and expressed in E. coli under cGMP manufacturing standards. MaxNuclease is a non-specific nuclease with high activity and specificity that degrades all forms of nucleic acids including single- and double-stranded, linear and circular nucleic acids.
What does MaxNuclease do?
DNA/RNA Degradation into 2-5 base oligonucleotides
MaxNuclease is a homodimer of two 30 kDa subunits containing two disulfide bonds that are essential for activity and stability. It hydrolyzes internal phosphodiester bonds between nucleotides in nucleic acids to produce 5′-monophosphate oligonucleotides of 2-5 bases in length.
What is MaxNuclease used for?
High-Efficiency Endonuclease for Biologics Manufacturing
Products such as lentiviral or AAV vectors, antibody drugs, vaccines, and recombinant protein drugs are expressed and produced by continuously passaged cells. Even after a fine purification process, host nucleic acids may remain in the products, and the residual nucleic acids may cause pathogenicity, tumorigenesis, etc. Residual nucleic acids need to be removed to a safe level in the final drug product.
Product Features
Manufactured in a GMP-compliant facility
Raw materials free from animal-derived components
Low Endotoxin: ≤ 0.01 EU/kU
Strict quality management for clinical manufacturing
FDA Drug Master Files: DMF #036799
Product Specifications
| Parameter | Specification |
| Catalog # | GMP-NUC-SE101 |
| FDA Drug Master Files | #36799 |
| Source | E. coli with endonuclease gene from Serratia marcescens |
| Molecular Weight | Approximately 27.8 kDa |
| Formulation | 20mM Tris-HCl, 20mM NaCl, 2mM MgCl2, 50% Glycerol, pH 8.0 |
| Storage | Store at -20±5°C. Avoid repeated freeze-thaw. |
| Activity | ≥250 U/μL analyzed by degradation of Salmon Sperm DNA |
| Unit Definition | One unit corresponds to the amount of enzyme required to produce a change in absorbance at 260 nm of 1.0 in 30 minutes, at 37°C and pH 8.0. |
Quality Control Criteria
| Assay | Specification |
| Activity (Dissolve herring sperm DNA) | ≥ 250 U/µL |
| Purity (Bis-Tris) | ≥ 95% |
| Purity (SEC-HPLC) | ≥ 99% |
| Residual Protease | Negative |
| Residual Host Protein | ≤ 10 ppm |
| Endotoxin | ≤ 0.01 EU/kU |
| Sterility | Negative |
| Residual Heavy Metal | ≤ 10 ppm |
| Mycoplasma | Negative |
Product Performance Validation
Host Cell DNA Removal
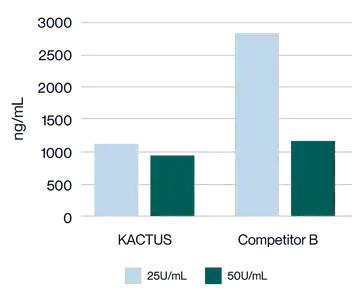
Virus harvest solution was treated with 25U/mL and 50U/mL endonuclease at 37°C for 2 hours, respectively. Detection of Host Cell DNA (HCD) residue was analyzed. KACTUS MaxNuclease has higher degradation activity versus Competitor B demonstrated by lower HCD residue for both 25U/mL and 50U/mL working concentrations.
Plasmid DNA Removal
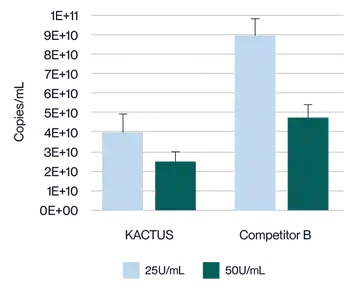
Virus harvest solution was treated with 25U/mL and 50U/mL endonuclease at 37°C for 2 hours, respectively. Detection of plasmid DNA (pDNA) residue was analyzed. KACTUS MaxNuclease has higher degradation activity versus Competitor B demonstrated by lower pDNA residue for both 25U/mL and 50U/mL working concentrations.
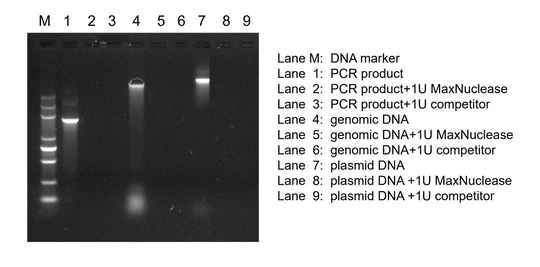
Degradation of PCR Product, Genomic DNA, and Plasmid DNA
MaxNuclease added to PCR product, genomic DNA, and plasmid DNA shows comparable degradation activity of nucleic acids to leading competitors.
Practical Applications of MaxNuclease™
MaxNuclease is identified from Serratia marcescens and is genetically engineered and expressed in E. coli under cGMP manufacturing standards. MaxNuclease is a non-specific nuclease with high activity and specificity that degrades all forms of nucleic acids including single- and double-stranded, linear and circular nucleic acids.
Our GMP Compliance
KACTUS MaxNuclease has been developed and verified with reference to cGMP production standards. The manufacturing process is in accordance with GMP production standards to ensure the traceability of raw materials in the production process. At present, this product has also been filed with the U.S. FDA Drug Master Files (DMF #036799).
Quality Management System
→ ISO13485 Accreditation
→ Digital Manufacturing Execution System (MES)
→ Process and Analytical method validation
→ Batch-to-batch stability and consistency
→ Free from antibiotic residues and raw materials of animal origin
→ Comprehensive records for batch production
→ Pharmaceutical Class A & C Clean Room
→ Validated and maintained equipment
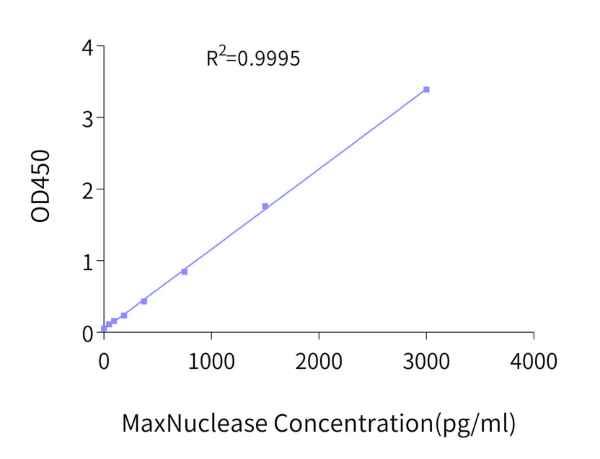
MaxNuclease ELISA Kit
After the nucleic acid is removed by MaxNuclease for biological products such as viral vectors and vaccines, it is also necessary to evaluate the residual MaxNuclease in the system. To this end, KACTUS has carefully developed a highly sensitive and specific residue detection kit, MaxNuclease ELISA Kit, with a sensitivity of up to 23 pg/mL.
MaxNuclease: FAQs
About MaxNuclease
What is MaxNuclease?
MaxNuclease, a broad-spectrum nuclease, is derived from Serratia Marcescen. It is expressed by genetically engineered E.coli and purified, all in a GMP-Grade environment. This enzyme can degrade all forms of DNA and RNA, including single-stranded, double-stranded, linear, circular, native, and denatured nucleic acids. It digests them into 5′-monophosphate oligonucleotides of 3-5 bases in length, and has no base recognition specificity. MaxNuclease can maintain high stability and enzyme activity under a wide range of conditions and is suitable for removing nucleic acid residues in samples and improving product purity in pharmaceutical industries such as viral vaccines, viral vectors, and recombinant proteins.
What are the main applications of MaxNuclease?
1) Removing DNA/RNA from biological products. The US FDA requires that the nucleic acid content of each dose of biological products for therapeutic use be less than 10pg. MaxNuclease can be used for the removal of nucleic acids in industrial biological products such as vaccines, polysaccharides, and proteins, so that the final nucleic acid content of biological products can meet regulatory requirements and the efficacy of biological products can be improved.
2) Purifying cell culture-derived products. Nucleic acids easily adhere to the surface of cell-generated particles such as virus-like particles (VLP), virus particles, and inclusion bodies, which changes the size or charge of the particles and causes the aggregation of these particles, such as during the storage of peripheral blood single cells (PBMC) agglomeration phenomenon. MaxNuclease can effectively degrade nucleic acid, avoid the influence of nucleic acids on cell products and purification, and help improve the purification efficiency of cell products.
3) Reducing the viscosity of lysed cells. MaxNuclease can degrade all forms of nucleic acid, reduce the viscosity of cell lysate, increase protein yield, improve separation effect, make it easy to filter (especially ultrafiltration), and facilitate downstream chromatographic purification operations.
4) Preparing samples for biochemical analysis. In analyses such as ELISA, chromatography, two-phase electrophoresis, and footprinting analysis, treatment of protein samples containing nucleic acids with MaxNuclease can improve resolution and improve recovery.
What are the main parameters of the MaxNuclease ELISA kit?
KACTUS’ MaxNuclease detection kit uses the double-antibody sandwich method to determine the content of MaxNuclease. Both the detection antibody and the coating antibody are recombinantly expressed monoclonal antibodies. The detection antibody is pre-coupled with HRP, which simplifies the operation steps and saves time. The kit has a sensitivity of 23.44 pg/mL and a linear range of 46.88 pg/mL-3000.00 pg/mL.
Usage
How stable is MaxNuclease?
We have tested the stability experiments at 25°C and 37°C. It was found that MaxNuclease can still maintain >90% of the enzyme activity under these two conditions for 7 days, which proves that the enzyme is very stable in a suitable storage buffer. Contact us for stability data.
What are the transport and storage conditions of MaxNuclease?
Store at -20°C. Avoid repeated freezing and thawing. The enzyme is valid for at least 2 years. We recommend shipping on dry ice.
At which step in the production process is MaxNuclease used?
MaxNuclease can be used for a variety of purposes (see “What are the main applications of MaxNuclease?”). Its usage and dosage vary according to the purpose of use. For the production of biological products such as viral vaccines, viral vectors, recombinant proteins, etc., MaxNuclease is generally added after harvesting and before purification.
What concentration of MaxNuclease should I use and how should I optimize the reaction conditions?
This depends on your application scenario and experimental condition. Generally, 25-50U/mL is suitable in the cell lysis step of an AAV production process. Due to different buffers and application scenarios, there will be relatively large differences in enzyme activity and optimal concentrations. For example, if the temperature is lower than 37°C during use, the enzyme activity will decrease. Compared to 37°C, MaxNuclease maintains about 22% of the enzyme activity at 4°C. When the temperature is lower than 37°C, the same nucleic acid removal effect can be achieved by appropriately extending the reaction time. Temperature, nuclease concentration, and time are the three conditions that mainly affect enzyme activity, and these three aspects can be optimized in practical applications.
What conditions are necessary to maintain the enzyme activity of MaxNuclease?
1-2mM Mg2+ is necessary to maintain enzyme activity. The reaction system can be adjusted by adding additional Mg2+ to maintain enzyme activity.
What experimental conditions inhibit enzyme activity?
Monovalent cations can inhibit enzyme activity, such as >300mM Na+, K+, or >100mM ammonium sulfate precipitation agent. Additionally, >1mM EDTA will inhibit enzyme activity because EDTA will chelate Mg2+ ions necessary for enzyme activity. Denaturing agents such as urea, as well as proteases, will also inactivate the enzyme.
How should I remove MaxNuclease from the final product?
MaxNuclease is easily removed in downstream purification with techniques such as depth filtration and tangential flow filtration (TFF). In pharmaceutical manufacturing, endonucleases are often removed by ion exchange chromatography. The PI of MaxNuclease is 6.85, and anion exchange chromatography is generally used to make the MaxNuclease flow through or be eluted.
In addition, the residue of MaxNuclelase is also part of the quality control analysis of pharmaceutical products. Generally, an ELISA kit can be used to detect MaxNuclease (including active and inactive) remaining in the product. The MaxNuclease detection kit developed by KACTUS uses a double antibody sandwich method to accurately quantify the residue of MaxNuclease in the product, with a sensitivity of 23.44 pg/mL and a linear range of 46.88 pg/mL-3000.00 pg/mL.
What is the enzyme activity of MaxNuclease and how is it measured?
One unit of activity corresponds to the amount of enzyme required to change the absorbance at 260 nm by 1.0 under the condition of excess salmon sperm DNA at 37°C and pH 8.0 for 30 minutes (equivalent to the amount of enzyme required to completely digest 37ug of DNA substrate). The verified enzyme activity of MaxNuclease is ≥ 250 U/µL.
Quality
What supporting documents can KACTUS provide for MaxNuclease?
Documents such as product specification, COA, COO, MSDS, TSE/BSE declaration can be provided. This product has passed the FDA DMF Type II record, with DMF #036799.
Does KACTUS support a reliable, bulk supply of MaxNuclease?
Yes, with our prokaryotic fermentation system and supernatant expression, there is no need for renaturation, which allows us to avoid mixing intermediate renaturation products. Additionally, high expression levels allow for large-scale individual batches to meet manufacturing production requirements.
Is MaxNuclease manufactured GMP-Grade?
Yes, MaxNuclease is manufactured in our dedicated GMP-Grade production facility and undergoes strict quality release testing. Additionally, we provide customizable documentation for regulatory filings. MaxNuclease has also been submitted to the FDA Drug Master Files (DMF #036799).
DNA Amplification & Modification Enzymes
Robust isothermal DNA amplification.
Available Products
Phi29 DNA Polymerase (#PHI-BE101)
Phi29 DNA polymerase is a highly processive enzyme that can efficiently amplify DNA sequences in vitro. It is often used as an alternative to the more commonly used polymerase chain reaction (PCR) method for DNA amplification. Phi29 DNA polymerase amplification has several advantages over PCR, including higher processivity, reduced reaction time, and higher fidelity. It is particularly useful for amplifying DNA from samples with limited starting material, such as ancient DNA or single cells. It is a highly robust enzyme and is suitable for rolling circle amplification (RCA), multiple-displacement amplification (MDA), and whole genome amplification.
Features
Isothermal amplification
High sensitivity
High processivity
High fidelity
Amplification with low starting quantities
Applications
Whole genome amplification
Single-cell genome sequencing
Rolling circle amplification
DNA manufacturing
Replication requiring isothermal or moderate temperature amplification
TelN Protelomerase (#TLN-BE001)
TelN Protelomerase cleaves dsDNA at its recognition site and leaves covalently closed ends. It can be used for DNA vaccine development as well as for non-viral therapy vectors. TelN Protelomerase is isolated from phage N15.
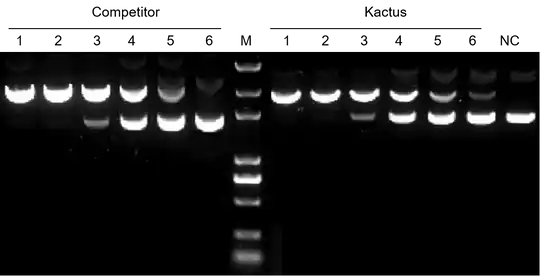
TelN Protelomerase efficiently converts plasmid DNA into linear DNA with hairpin ends.
Exonuclease III (#EXO-EE101)
Exonuclease III is a highly processive enzyme that hydrolyzes the phosphodiester bonds in DNA in the 3′ to 5′ direction, resulting in stepwise removal of nucleotides. Exonuclease III catalyzes the removal of nucleotides from linear or nicked double stranded DNA. Degradation of Exonuclease III could be initiated from a 3’ blunt end, 3’ recessed end, 3’ overhangs with less than 4 bases and nicked DNA.

References
[1] Technical Guidance Principles for the Pharmacological Research and Evaluation of In Vivo Gene Therapy Products (Trial)
[2] Savić N, Schwank G. Advances in therapeutic CRISPR/Cas9 genome editing. Transl Res.