Recombinant Proteins
Products
Overview
Since the approval of the first antibody drug OKT3 in 1986, antibody drugs have occupied an important position in the pharmaceutical market. Currently, antibody drugs mainly come in various types such as monoclonal antibodies, bispecific antibodies, and XDCs (such as antibody-drug conjugates, ADCs). With the continuous development and improvement of monoclonal antibody technology, antibody drugs have become highly diversified in terms of target types, drug forms, and therapeutic areas. KACTUS, with its proprietary protein research and development production platform SAMS™, has launched various types of high-quality recombinant proteins to fully meet customer needs for antibody development.
Target Proteins for Antibody Discovery
In the early stages of antibody drug development, it is often necessary to use antigen proteins for animal immunization to obtain antibodies. KACTUS offers a wide variety of target proteins, all of which have undergone strict biological activity tests, aiding customers in animal immunization. Example data:
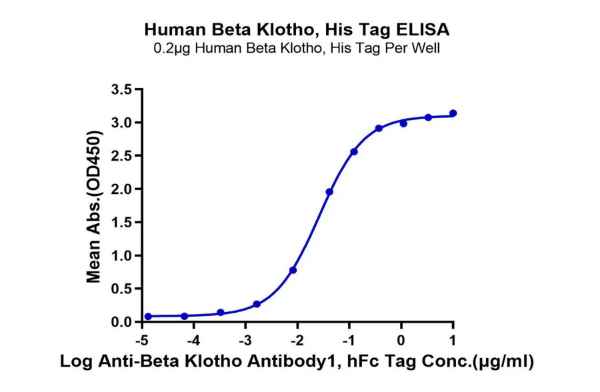
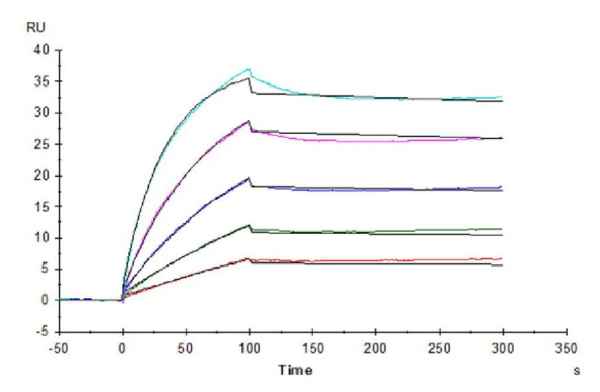
Figure 1. Our recombinant human β-Klotho protein exhibits excellent binding ability with its antibody (left graph, ELISA) and ligand FGF21 (right graph, SPR).
For multiple transmembrane protein targets, such as the GPCR family and the Claudin family, the presence of several hydrophobic regions makes it difficult to be solubly expressed and maintain activity. KACTUS adopts special designs, such as using virus-like particle (VLP) proteins for display, allowing soluble expression while maintaining the correct conformation and biological activity of the protein. Additionally, for proteins with poor direct immunization effects or weak immunogenicity, utilizing VLP display not only retains good biological activity but also achieves stronger immunization effects.
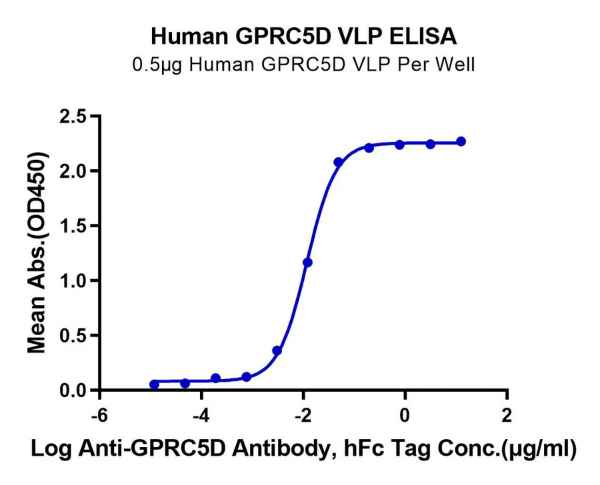
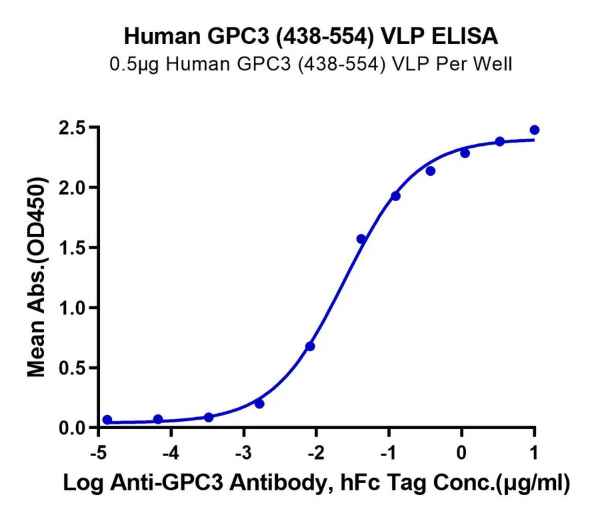
Figure 2. GPRC5D and GPC3, displayed using VLP, both exhibit good binding activity with their respective antibodies.
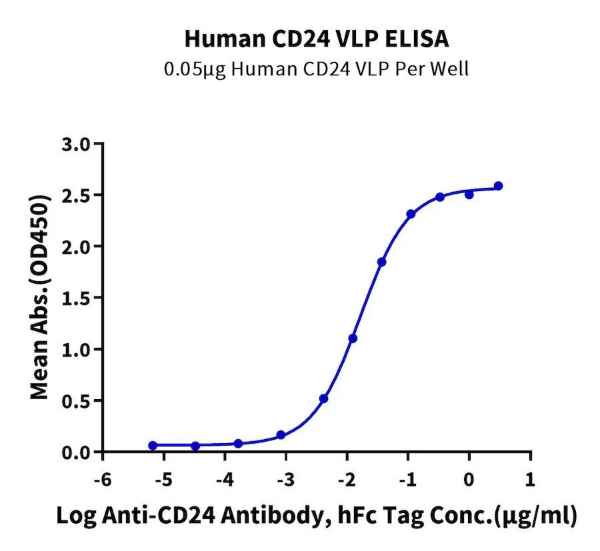
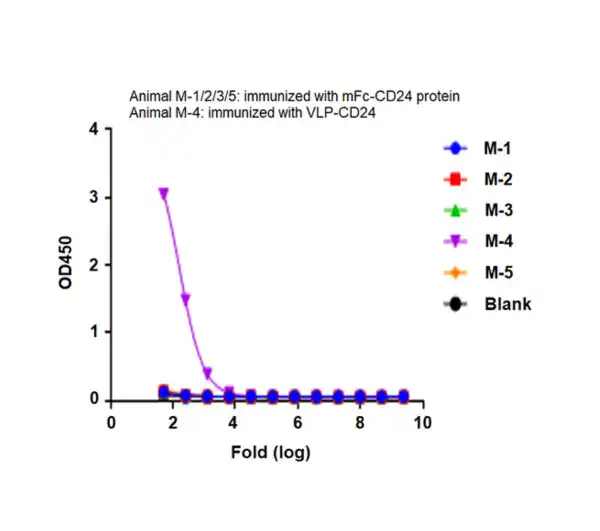
Figure 3. CD24, displayed using VLP, not only binds well with the antibody (left), but also shows a significant enhancement in immunization effects (right).
Biotinylated Target Proteins for Antibody Screening
After obtaining antibodies, it is necessary to screen and optimize the acquired antibodies. Proteins labeled with biotin have higher sensitivity and specificity, which can be used not only for various detection analyses but also for immune capture. They have unique advantages in analyses like ELISA, SPR, flow cytometry, and biopanning.
KACTUS offers both site-specific and non-site-specific biotinylated proteins for customers to conduct antibody screening tests. Site-specific biotinylated proteins utilize the principle that the lysine residue on the Avi tag can be modified with biotin by the biotin ligase BirA, enabling precise control of biotin modification. Moreover, when the protein is fixed to the surface of the affinity-coated bead, it has directional consistency and is widely used in various detection analyses.
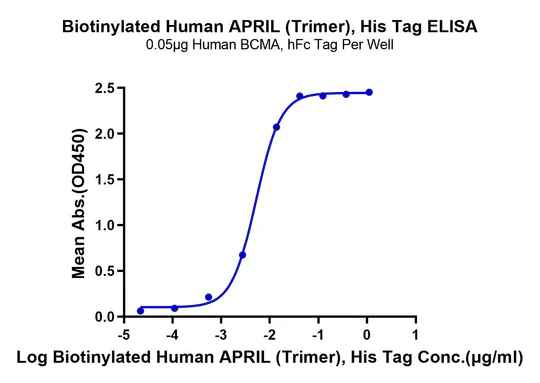
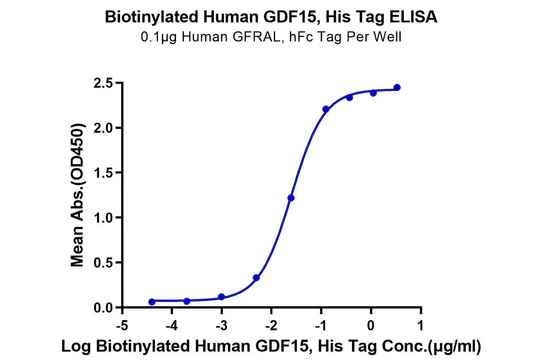
Figure 4. Verified by ELISA, site-specific biotin-modified Human APRIL (Trimer) (left) and GDF15 (right) both exhibit good binding activity with their respective ligands.
Non-site-specific biotinylated recombinant proteins are produced by chemical labeling, where the free amino groups or lysine residues of the protein can bind with biotin. This method may have higher sensitivity as multiple biotins can be labeled on each protein molecule.
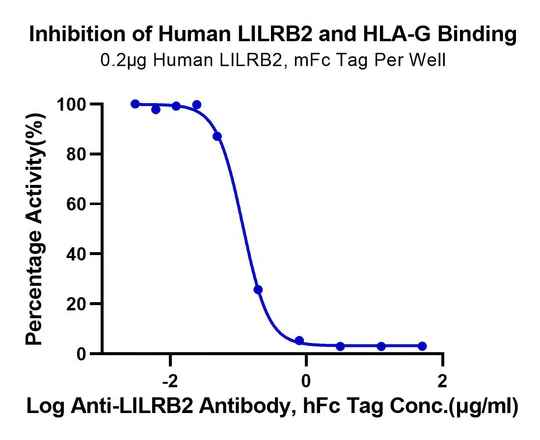
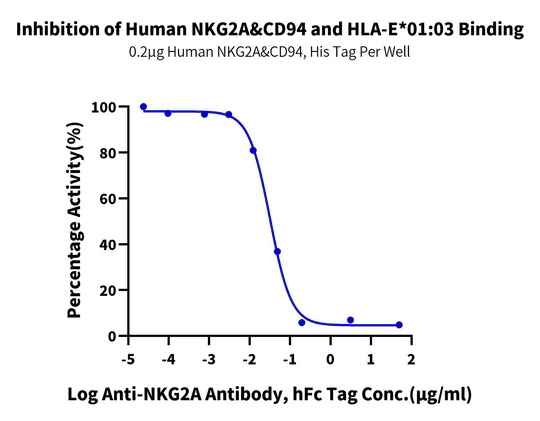
Figure 5. Verified by ELISA, non-site-specific biotinylated labeled HLA-G or HLA-E tetramers can be applied in blocking analysis experiments related to corresponding antibodies.
In addition, during the performance evaluation stage of antibody drugs, besides analyzing the binding situation between the antibody Fab fragment and the target, it is also necessary to analyze the binding ability of the Fc segment with the Fc receptor, to evaluate the antibody’s half-life or the antibody’s ADCC effect. The Fc receptor protein series from KACTUS, including FcRn and various mutated forms of Fcγ receptors, can aid in antibody optimization and in obtaining antibodies with the desired affinity.
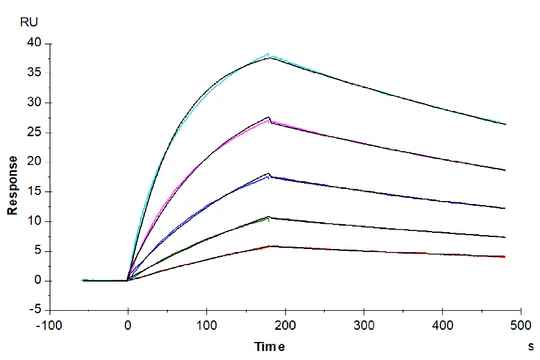
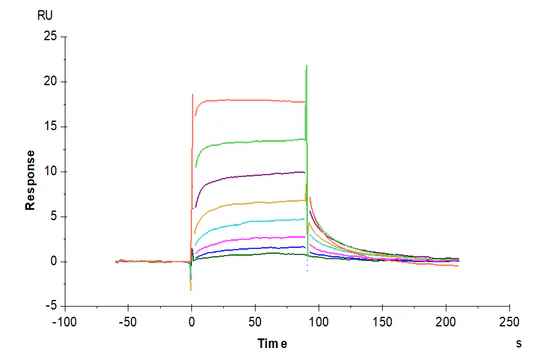
Figure 6. (Left) Verified by SPR, Trastuzumab can bind with Human Fc gamma RI, with an affinity constant of 1.94 nM. (Right) Verified by SPR, Human IgG1 can bind with Human FcRn, with an affinity constant of 0.28 µM.
Target Proteins for CMC Method Development
CMC (Chemistry, Manufacturing, and Controls) is one of the key links for the successful development and registration of a drug, requiring high-quality proteins with high stability to establish production control parameters and imposing higher requirements on the stability and activity of the proteins. KACTUS’ recombinant protein products possess high stability and excellent activity, which can be applied in the CMC method development of antibody drugs. Example data:

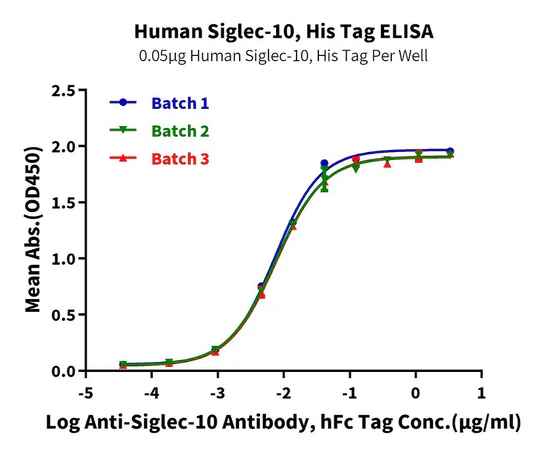
Figure 7. Verified by ELISA, recombinant Human Trop-2 (left) and Siglec-10 (right) proteins exhibit good batch-to-batch stability.


Figure 8. Verified by SPR, both biotinylated Claudin 6-VLP (left) and biotinylated CD20-VLP (right) proteins can bind well with their respective antibodies.
Custom Target Protein Production
To cater to diverse and specialized requirements, KACTUS offers custom target protein production. We are committed to delivering a spectrum of target proteins and associated analytical services like SPR analysis, aligning with the intricate needs of antibody drug development endeavors of our clients.
Our refined and structured approach to developing customized target proteins includes:
Confidentiality Agreement
Technical Discussion & Protein Design
Expression Testing
Activity Testing
Antibody therapy has emerged as one of the most rapidly advancing treatment modalities, with innovative antibody discovery technologies fast-tracking the progression of antibody drugs into clinical stages. As an increasing number of antibody drugs receive approval, there is renewed hope for cancer patients for extended life expectancy and enhanced quality of life. KACTUS remains steadfast in its commitment to providing premium recombinant protein products, serving as a pillar of support for antibody drug research, with the ultimate goal of facilitating swift benefits to patients.
Features of KACTUS Custom Recombinant Proteins
Choice of Species, Tag, Label, etc.
Highly Customizable
Batch-to-Batch Stability
High Purity
Proven Biological Activity
Strict Endotoxicity Control
VLP & Nanodisc Multi-Pass Transmembrane Proteins
Background
KACTUS offers a specialized range of VLP (virus-like particle) and Nanodisc transmembrane proteins, designed for accurate representation of multi-pass transmembrane proteins in their native conformation. These full-length proteins are produced using detergent-free extraction methods, ensuring the preservation of structural integrity and biological activity. To support a wide range of applications, including ELISA, flow cytometry, and SPR, our transmembrane proteins can be biotinylated or fluorescently labeled, offering flexibility for research needs. Additionally, we test our VLPs and Nanodiscs for bioactivity through SPR, ELISA, and flow cytometry, ensuring batch consistency and reliability. KACTUS’ VLP and Nanodisc products are valuable tools for studies in antibody discovery, antibody screening, analytical method development, and immunization.
Technology Platforms
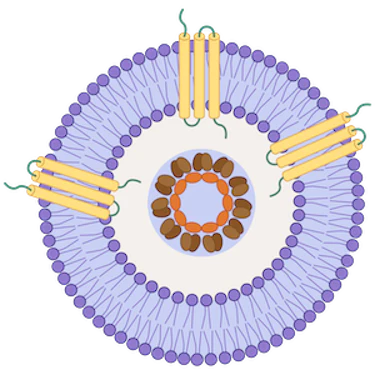
Virus-Like Particle (VLP)
Mammalian-expressed transmembrane proteins displayed on VLPs for native conformation and boosted immunogenicity
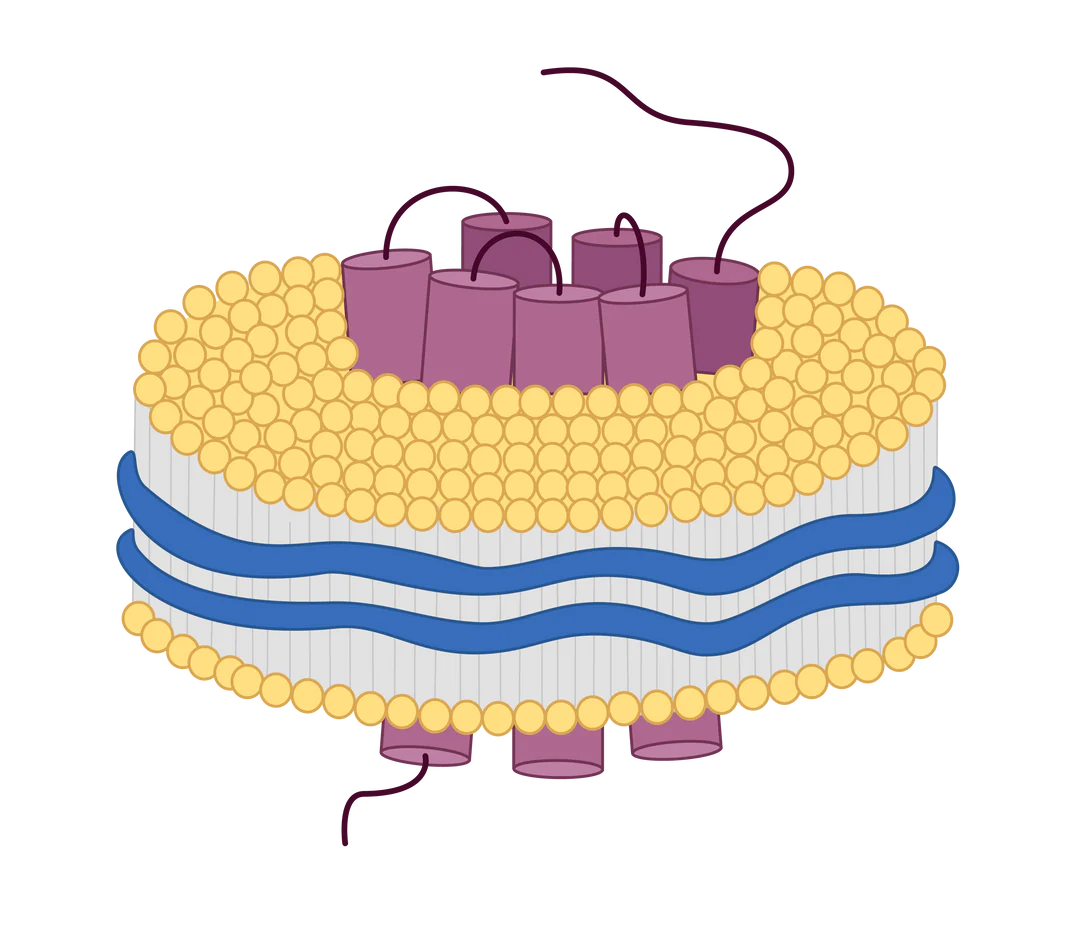
Nanodisc
Transmembrane proteins expressed on membrane-like structures composed of phospholipids and copolymers
Virus-like particles (VLPs) are small nanoparticles composed of the structural proteins of a virus, specifically the shell or capsid proteins. These proteins self-assemble into virus-like structures, mimicking the organization and morphology of the actual virus. However, VLPs are devoid of the viral infectious genomes, rendering them non-infectious and relatively safe for use in various applications, including vaccine development and gene therapy.
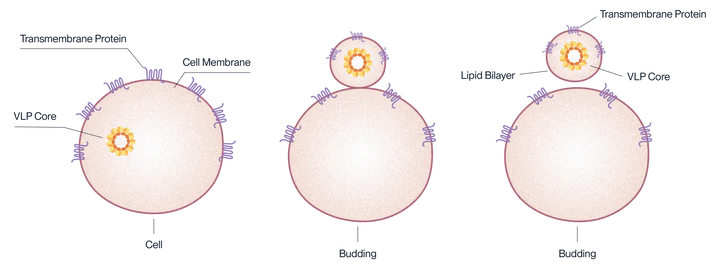
Mechanism of expression for VLP-displayed antigens.
Why use VLP-displayed antigens?
Virus-Like Particles (VLPs) are good at boosting the immune response. This makes them useful for immunization, especially with antigen targets that are usually present in low amounts or don’t trigger a strong immune reaction by themselves. They also allow for soluble expression of multipass transmembrane proteins in their natural configuration.
Applications
→ Blood sample determination: ELISA
→ In vivo pharmacokinetic analysis
→ Antibody Immunization, Screening, Functional Characterization
→ CMC method development
→ Affinity determination: ELISA, SPR
Features
→ Site-Specific Biotinylation
→ VLP framework optimized to each antigen
→ Proven biological activity via ELISA and SPR
→ Mammalian cell expression system for natural folding and glycosylation
→ Boosted immunogenicity for antibody drug discovery
Product Validation Data
Claudin 18.2 VLP
In 2018, KACTUS became the first company globally to express full-length wild-type Claudin 18.2, a multi-transmembrane protein with significant implications for gastric and esophageal adenocarcinomas. Despite its potential, technical challenges in generating high-quality Claudin 18.2 antigen have previously hindered antibody isolation and production quality control. Leveraging our SAMS™ protein engineering and expression platform, we have successfully produced full-length Claudin 18.2 antigens on VLPs. Our robust product performance testing demonstrates the functional integrity and bioactivity of our VLP expression platform.
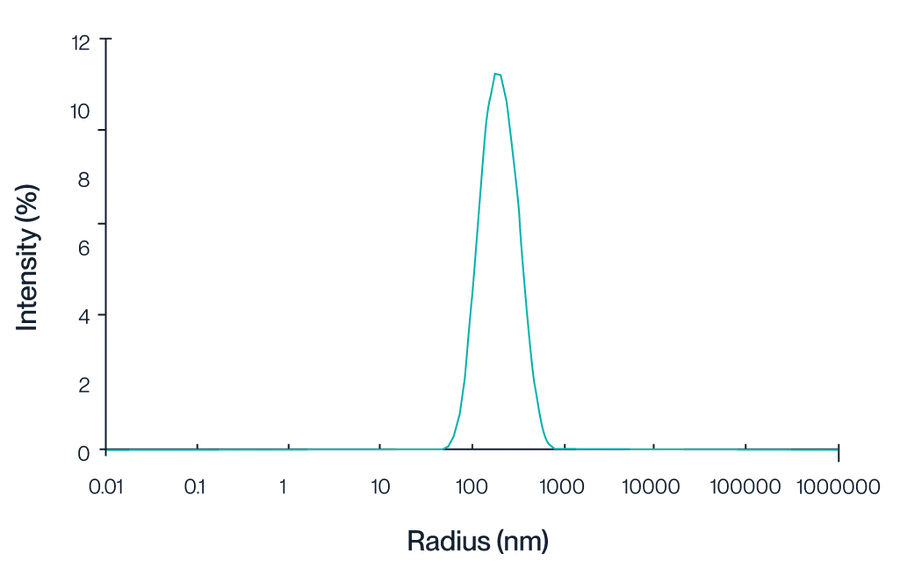
Figure 1. This graph illustrates the intensity distribution of Claudin 18.2 VLPs as measured by dynamic light scattering (DLS). The peak centered at approximately 150 nm indicates a homogeneous population of VLPs, reflecting the consistent size and quality of the particles.
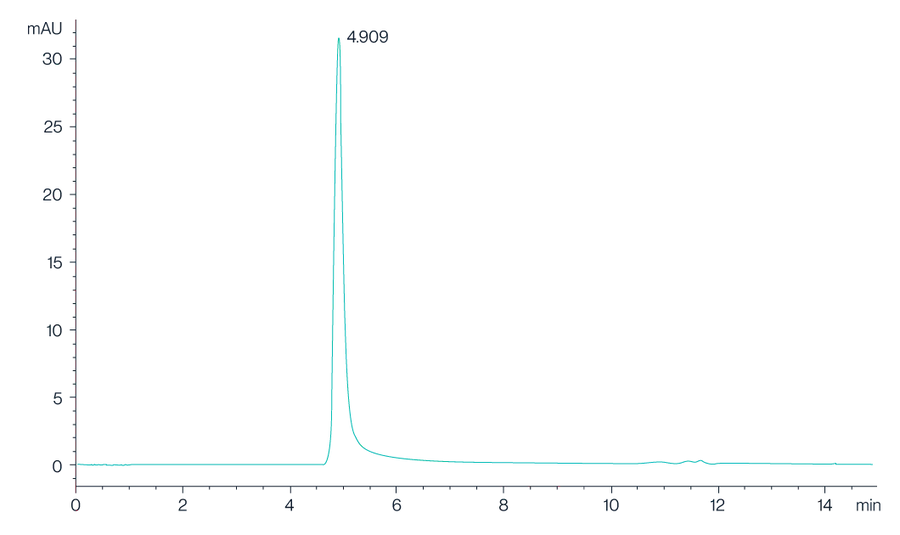
Figure 2. The chromatogram shows the high-performance liquid chromatography (HPLC) profile of Claudin 18.2 VLPs, with a prominent peak observed at 4.909 minutes, indicating the retention time of the Claudin 18.2 protein. The sharp and well-defined peak suggests a high purity and consistency of the VLP preparation.
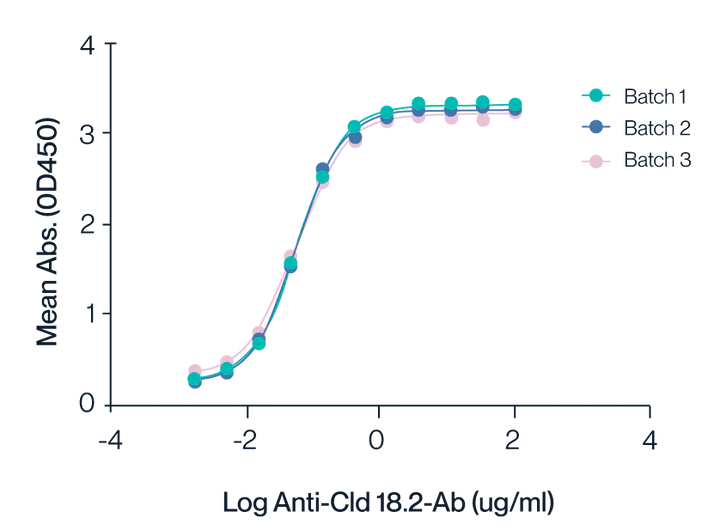
Figure 3. ELISA assay shows the binding activity of Claudin 18.2 VLPs to anti-Claudin 18.2 antibodies across three different production batches. The consistent curves for Batches 1, 2, and 3 highlight the reproducibility and stability of the VLP preparations. The half-maximal effective concentration (EC50) is determined to be 0.1 nM, indicating high affinity and robust interaction between Claudin 18.2 VLPs and the specific antibodies.
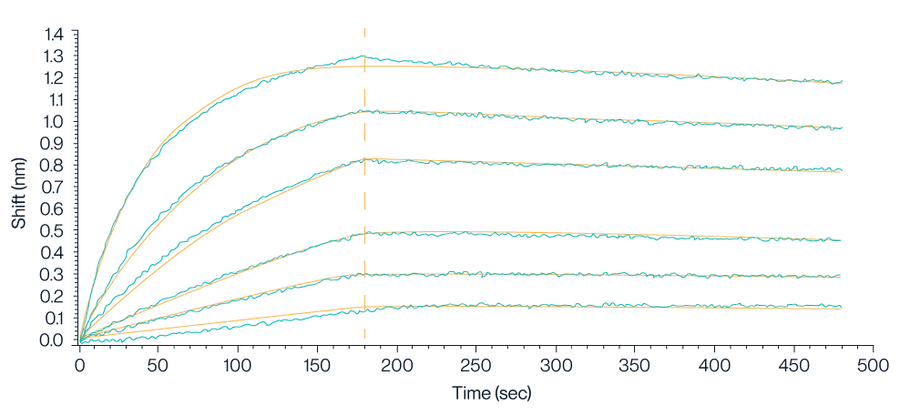
Figure 4. Biolayer Interferometry (BLI) data demonstrates the binding kinetics of Claudin 18.2 VLPs to streptavidin-labeled probes. The analysis reveals a high binding affinity with a dissociation constant (KD) of approximately 5.73E-10 M, indicating strong and stable interaction.
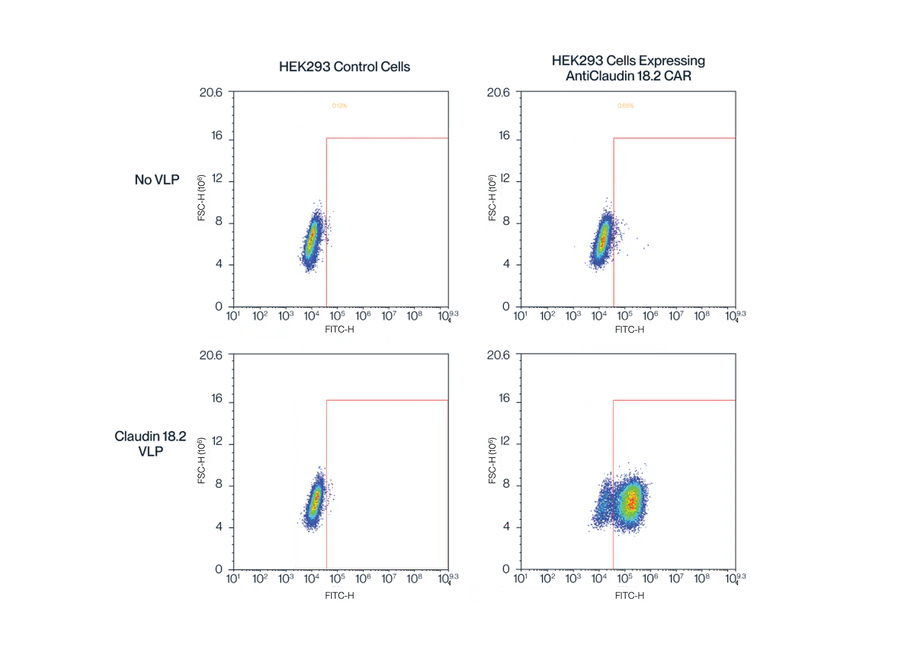
Figure 5. Flow cytometry data demonstrates the binding specificity and quantification of Claudin 18.2 displayed on VLPs. The top panels show the results from HEK293 control cells, while the bottom panels show the results from anti-Cld18.2 CAR-expressing HEK293 cells. The presence of Cld18.2 VLPs results in a significant shift in the fluorescence signal in anti-Cld18.2 CAR-expressing cells, indicating successful detection and quantification of Cld18.2 on VLPs. This validates the high-quality expression and bioactivity of the Claudin 18.2 VLPs.
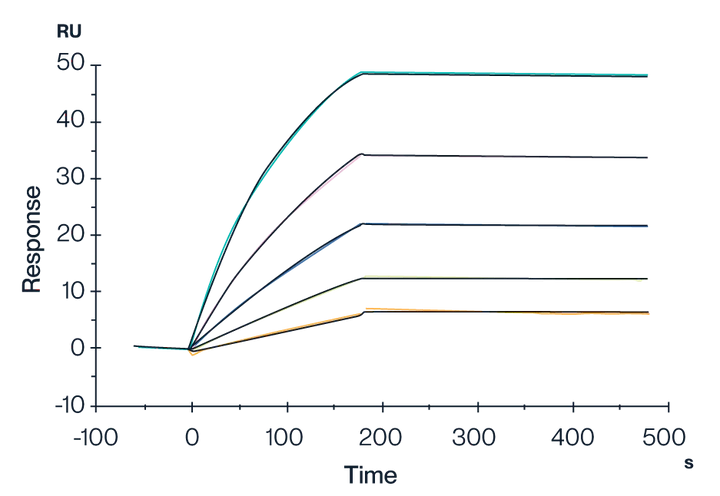
Figure 6. SPR data showing the binding interactions of Biotinylated Claudin 18.2 VLPs with streptavidin immobilized on a CM5 chip. Results show a dissociation constant (KD) of 1.638E-11 M. This indicates a very strong and stable binding affinity of the Claudin 18.2 VLP.
VLP Products
| Description | Product Size | Catalog No. |
| VLP Control | 100UG/500UG | VLP-HM00C |
| Human Claudin 6 Protein-VLP | 100UG/500UG | CLD-HM006 |
| Human CD20 Protein-VLP | 100UG/500UG | CD2-HM122 |
| Human GPRC5D Protein-VLP | 100UG/500UG | GPR-HM05P |
| Biotinylated VLP Control | 100UL | GPR-HM05CB |
| Biotinylated Human GPRC5D Protein-VLP | 100UL | GPR-HM05PB |
| Human Claudin 9 Protein-VLP | 100UG/5X100UG | CLD-HM009 |
| Cynomolgus GPRC5D Protein-VLP | 100UG/500UG | GPR-CM05P |
| Human GPC3 (438-554) Protein-VLP | 100UG/500UG | GPC-HM003 |
| Biotinylated Human Claudin 6 Protein-VLP | 100UL | CLD-HM006B |
| Cynomolgus Claudin 6 Protein-VLP | 100UG/500UG | CLD-CM006 |
| Mouse Claudin 6 Protein-VLP | 100UG/500UG | CLD-MM006 |
| Human CCR2b Protein-VLP | 100UG/500UG | CCR-HM02B |
| Human Claudin 4 Protein-VLP | 100UG/500UG | CLD-HM104 |
| Human GPC3 Protein-VLP | 100UG/500UG | GPC-HE005 |
| Mouse GPRC5D Protein-VLP | 100UG/500UG | GPR-MM05P |
| Human TM4SF1 Protein-VLP | 100UG/500UG | TSF-HM002 |
| Human SSTR2 Protein-VLP | 100UG/500UG | STR-HM002 |
| Human CD24 Protein-VLP | 100UG/500UG | CD2-HM124V |
| Human CD24 Protein-VLP | 500UG/500UG | CD2-HM124V |
| Cynomolgus CD24 Protein-VLP | 100UG/500UG | CD2-CM124V |
| Cynomolgus CD24 Protein-VLP | 500UG/500UG | CD2-CM124V |
| Biotinylated Human CCR2b Protein-VLP | 100UL | CCR-HM02BB |
Nanodiscs are membrane-like structures composed of phospholipids and membrane scaffold molecules, which are typically synthetic copolymers or membrane scaffold proteins. We use a proprietary amphipathic copolymer to enable membrane protein stabilization & solubilization without the use of detergents.
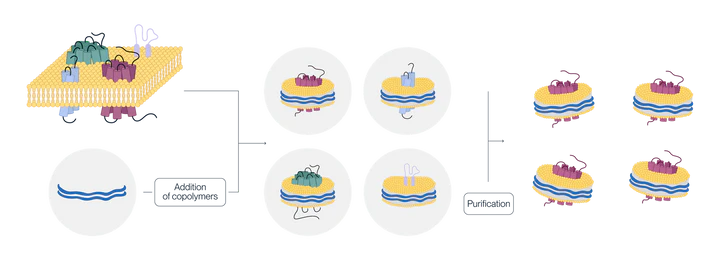
Schematic diagram of nanodisc displaying a multi-transmembrane protein
Why use nanodisc-displayed antigens?
Using Nanodisc technology to display multitransmembrane proteins not only better maintains their natural conformation but also addresses the issue of soluble preparation of these proteins. Moreover, because nanodiscs can be extracted without detergents, they offer a superior alternative for multitransmembrane proteins that are sensitive to maintaining activity with detergents.
Applications
→ Yeast display screening
→ In vitro functional assays
→ CAR expression testing
→ PK/PD Studies
→ Analytical Tests, ELISA, SPR, BLI
Features
→ Native structure and conformation
→ Detergent free extraction for reliable performance
→ Good water solubility
→ Proven biological activity via ELISA and SPR
→ Mammalian cell expression system for natural folding and glycosylation
Product Validation Data
GPRC5D Nanodisc
GPRC5D is an important multitransmembrane protein with significant therapeutic and research potential. To ensure the most accurate representation of its natural conformation and activity, we’ve developed a GPRC5D Nanodisc with detergent-free extraction. This innovative approach not only maintains the native structure of GPRC5D but also overcomes the challenges associated with the soluble preparation of multi-transmembrane proteins. We’ve performed extensive product quality testing to demonstrate the efficacy and quality of our Nanodisc-based antigens.
Additionally, to further support the development of antibody drugs (such as bispecific antibodies), we’ve developed biotinylated Nanodisc-displayed multitransmembrane proteins. These proteins exhibit high binding activity and can be applied to various research stages, including ELISA method development and pharmacokinetic analysis.
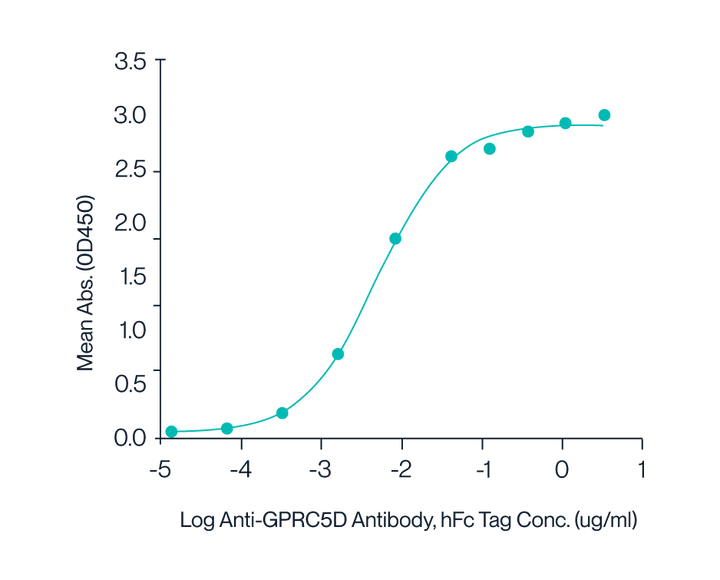
Figure 7. Human GPRC5D Nanodisc with His Tag was immobilized at 2 µg/ml (100 µl/well) on the plate. The dose-response curve for the Anti-GPRC5D Antibody with hFc Tag was generated, with an EC50 of 4.9 ng/ml as determined by ELISA.
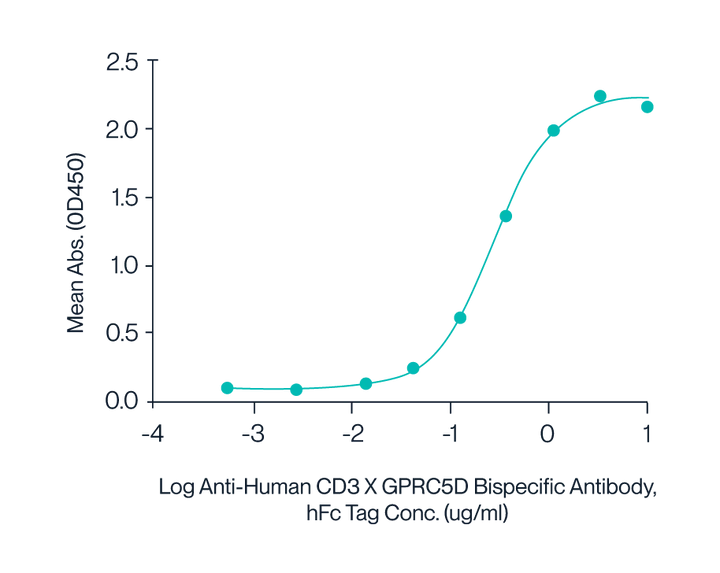
Figure 8. Human CD3E&CD3D, with a His Tag, was immobilized on the plate at a concentration of 2 μg/mL (100 μL/well). Serial dilutions of the Anti-Human CD3×GPRC5D bispecific antibody, with an hFc Tag, were then added, followed by the addition of biotinylated Human GPRC5D Nanodisc, with a His Tag, at 5 μg/mL. Detection was performed using HRP-conjugated streptavidin, and the EC50 was determined to be 0.28 μg/mL by ELISA.
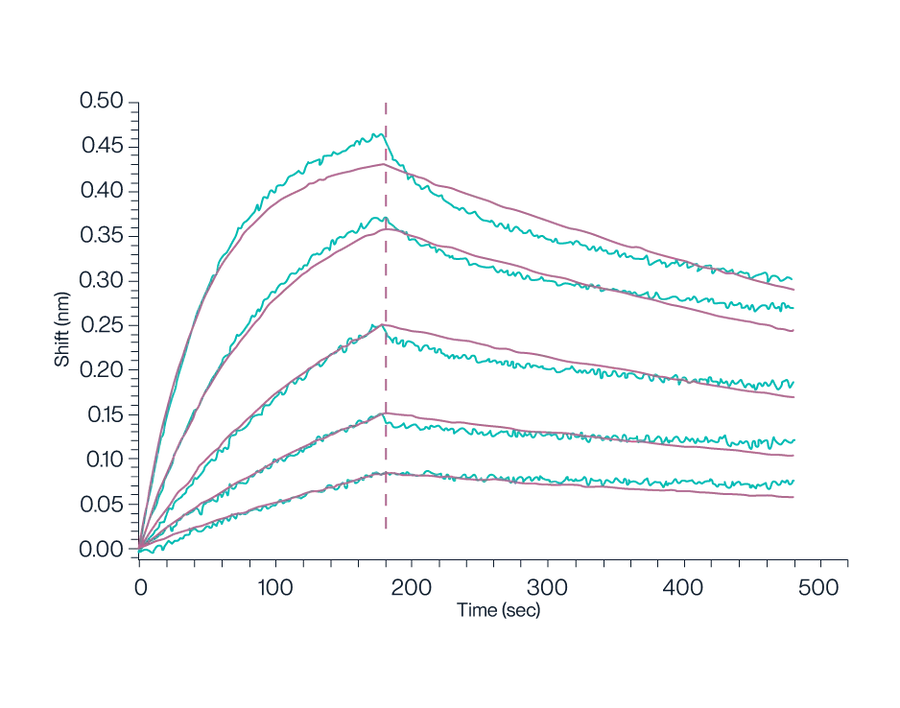
Figure 9. Biotinylated Human GPRC5D Nanodisc, with a His Tag, was loaded onto an SA-Biosensor. This setup was used to determine the binding affinity to the Anti-GPRC5D Antibody, which was measured to be 1.16 nM using a BLI assay.
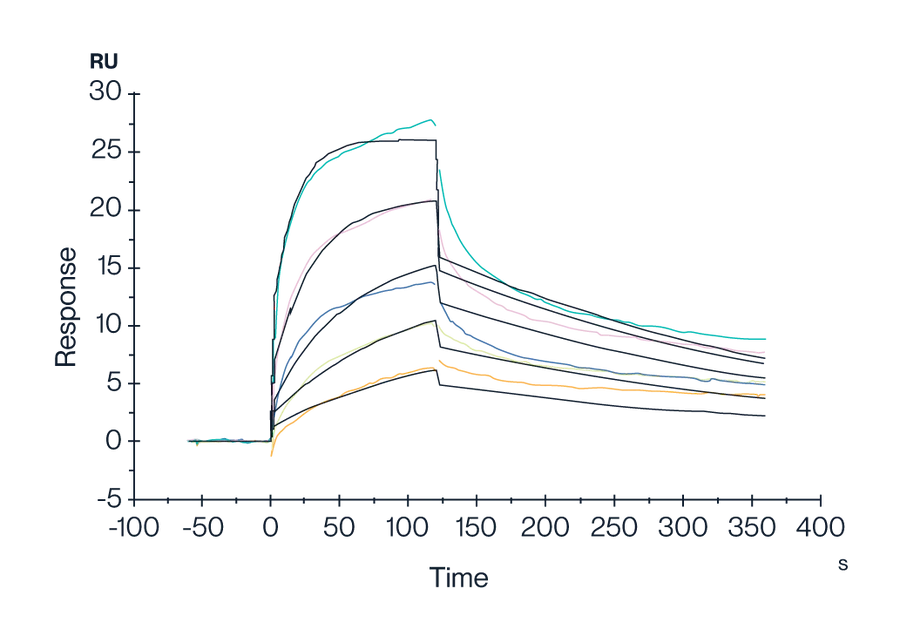
Figure 10. Human GPRC5D Nanodisc with His Tag was captured on a CM5 Chip via an anti-His antibody. It was found to bind the Anti-GPRC5D Antibody with an affinity constant of 1.47 nM, as determined by SPR assay (Biacore T200).
Nanodisc Products
| Description | Size | Catalog No. |
| Biotinylated Human GPRC5D Protein-Nanodisc | 100UG | GPR-HM45PB |
| Biotinylated Human GPRC5D Protein-Nanodisc | 500UG | GPR-HM45PB |
| Human GPRC5D Protein-Nanodisc | 100UG | GPR-HM15P |
| Human GPRC5D Protein-Nanodisc | 500UG | GPR-HM15P |
| Human SSTR2 Protein-Nanodisc | 100UG | STR-HM1N1 |
| Human SSTR2 Protein-Nanodisc | 500UG | STR-HM1N1 |
| Human A2AR Protein-Nanodisc | 100UG | A2R-HM1N1 |
| Human A2AR Protein-Nanodisc | 500UG | A2R-HM1N1 |
| Human LGR-4 Protein-Nanodisc | 100UG | LGR-HM10N |
| Human LGR-4 Protein-Nanodisc | 500UG | LGR-HM10N |
| FITC-equivalent Human CCR7 Protein-Nanodisc | 100UG | CCR-HM107 |
| FITC-equivalent Human CCR7 Protein-Nanodisc | 500UG | CCR-HM107 |
| Catalog No. | Type | Target | Species | Amino Acid Range | Tag | Express System |
| A2R-HM1N1 | Nanodisc | A2AR | Human | Met1-Ser412 | C-His | HEK293 |
| CCR-HM02BB | VLP | Biotinylated CCR2b | Human | Met1-Leu360 | HEK293 | |
| CLD-HM006B | VLP | Biotinylated Claudin 6 | Human | Met1-Val220 | HEK293 | |
| GPR-HM45PB | Nanodisc | Biotinylated GPRC5D | Human | Met1-Val345 | C-His-Avi | HEK293 |
| GPR-HM05PB | VLP | Biotinylated GPRC5D | Human | Met1-Val345 | HEK293 | |
| GPR-HM05CB | VLP | Biotinylated VLP Control | Human | HEK293 | ||
| CCR-HM02B | VLP | CCR2b | Human | Met1-Leu360 | HEK293 | |
| CCR-HM107 | Nanodisc | CCR7 | Human | Met1-Pro378 | C-His | HEK293 |
| CD2-HM122 | VLP | CD20 | Human | Met1-Pro297 | HEK293 | |
| CD2-CM124V | VLP | CD24 | Cynomolgus | Ser26-Gly57 | HEK293 | |
| CD2-HM124V | VLP | CD24 | Human | Ser27-Gly59 | HEK293 | |
| CLD-CM006 | VLP | Claudin 6 | Cynomolgus | Met1 – Val220 | HEK293 | |
| CLD-HM006 | VLP | Claudin 6 | Human | Met1-Val220 | HEK293 | |
| CLD-MM006 | VLP | Claudin 6 | Mouse | Met1-Val219 | HEK293 | |
| CLD-HM009 | VLP | Claudin 9 | Human | Met1-Val217 | HEK293 | |
| GPC-HM003 | VLP | GPC3 (438-554) VLP | Human | Arg438-Asn554 | HEK293 | |
| GPC-HE005 | VLP | GPC3 VLP | Human | Gly510-Asn554 | E.coli | |
| GPR-CM05P | VLP | GPRC5D VLP | Cynomolgus | Met1-Cys300 | HEK293 | |
| GPR-MM05P | VLP | GPRC5D VLP | Mouse | Met1-Leu344 | HEK293 | |
| GPR-HM15P | Nanodisc | GPRC5D | Human | Met1-Val345 | C-His | HEK293 |
| GPR-HM05P | VLP | GPRC5D | Human | Met1-Val345 | HEK293 | |
| LGR-HM10N | Nanodisc | LGR-4 | Human | Ala25-Asp951 | C-His | HEK293 |
| STR-HM1N1 | Nanodisc | SSTR2 | Human | Met1-Ile369 | C-His | HEK293 |
| STR-HM002 | VLP | SSTR2 | Human | Met1-Ile369 | HEK293 | |
| TSF-HM002 | VLP | TM4SF1 | Human | Met1-Cys202 | HEK293 | |
| VLP-HM00C | VLP | VLP Control | HEK293 |
KACTUS VLP-Displayed Antigens FAQs
Are the VLPs provided in liquid or lyophilized form?
KACTUS can provide VLPs in liquid or lyophilized form. Lyophilized format allows for the use of a custom buffer or adjuvant during screening or immunization as desired. It also ensures stability of the VLPs for use in a variety of settings, such as for screening or immunization. Our lyophilization team tests our catalog products for activity after lyophilization, to ensure you are still receiving a bioactive protein.
What are the size of the VLPs?
Generally, VLPs range in size from approximately 20 to 200 nanometers (nm). Their smaller size lets engineered VLPs focus the immune response on specific surface antigens, more so than cell-based immunization.
What is the copy number of the antigen per VLP?
Our VLPs are produced with high quality standards. Although it is difficult to determine the exact number of copies of the antigen displayed on the surface of the VLP, we take steps to validate each batch of VLPs to assure the displayed protein will have consistency across batches.
What quality control standards are used for the VLP-displayed antigens?
To guarantee VLP quality, KACTUS follows a strict validation process involving various analytical techniques for each batch. This process includes techniques such as size exclusion chromatography, ELISA, and SPR.
How do I order a VLP antigen?
Browse the catalog and order directly online via credit card or email us at info@stratech.co.uk to place an order via PO.
How do I order a custom VLP protein?
Contact us here to request a custom VLP protein.
What is the lead time for VLP antigens?
Our catalog products have a lead time of 2 days to 2 weeks. Custom orders have a turnaround time of 6-8 weeks. Contact us for more information.
Where can I get more information regarding VLP antigens?
Contact us to request more information or set up a meeting with one of our team members.
Highly Active CD3 Proteins for Advanced Immunization and Antibody Research Monomers, homodimers, heterodimers, and biotinylated proteins
Product Features
High Protein Affinity
We verified the activity of our heterodimers, such as CD3E/G-His Tag and CD3E/D-His Tag, via ELISA and SPR.
Equal Expression of Subunits
A proprietary design, expression, and purification system ensures the two subunits of the heterodimers are expressed in equal proportions with greater than 95% purity, verfied by SDS-PAGE and SEC-HPLC.
Stability
Our CD3 proteins have high batch-to-batch consistency and long-term stability as verified by ELISA.
CD3 Proteins Overview
About CD3 Proteins
CD3 (Cluster of differentiation 3) is one of the proteins expressed on the surface of T cells. It consists of six peptide chains, one γ chain, one δ chain, two ε chains and two ζ chains. These six peptide chains are all transmembrane, and the transmembrane region is connected with TCR through a salt bridge to form a tight TCR-CD3 complex, which jointly participates in the recognition and signal transduction of T cells to antigens. The activation signal generated by TCR recognition of antigen is transduced into T cells by the ITAM sequence in the cytoplasmic region of CD3, and then a series of immune responses are initiated.
Applications
Antibody Discovery
Immunization
Antibody Screening
Functional characterization
Affinity Determination (ELISA, SPR, BLI)
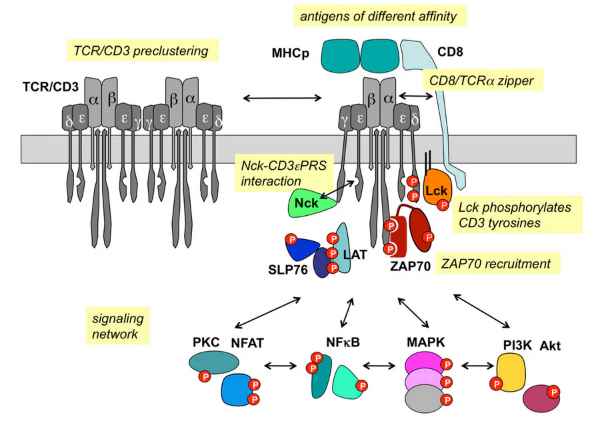
TCR/CD3 signaling (Louis-Dit-Sully C et. al, 2012)
Product Performance Validation
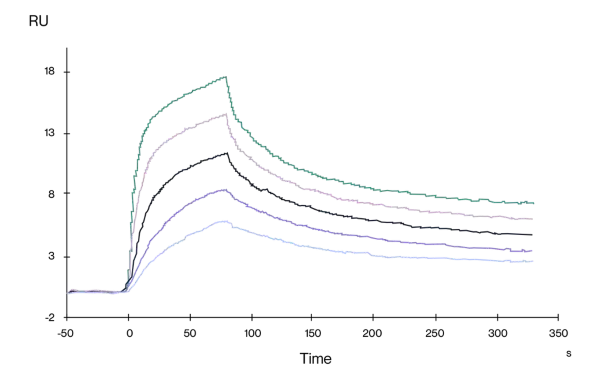
High Binding Affinity by SPR
Human CD3E/CD3D His tag, captured on CM5 chip via anti-His antibody, binds OKT3 mFc Tag with an affinity constant of 0.36nM as determined in SPR assay (Biacore T200).
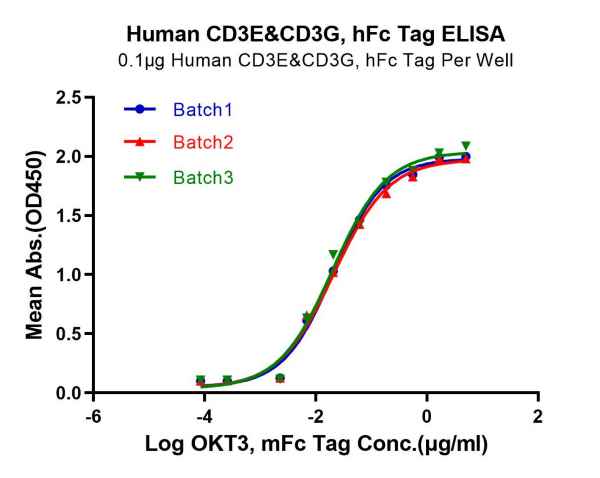
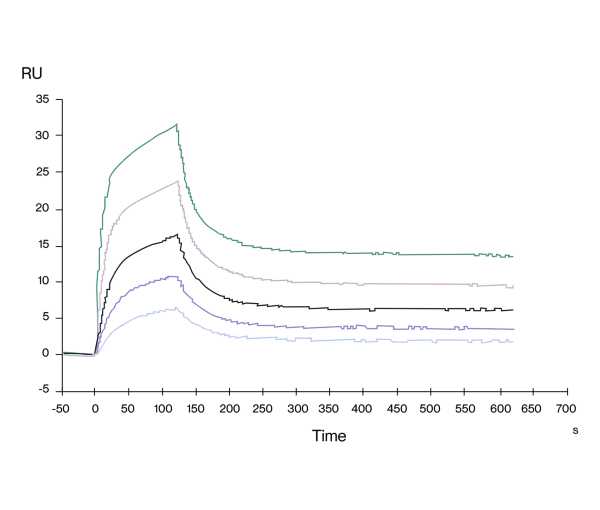
Batch-to-Batch Consistency
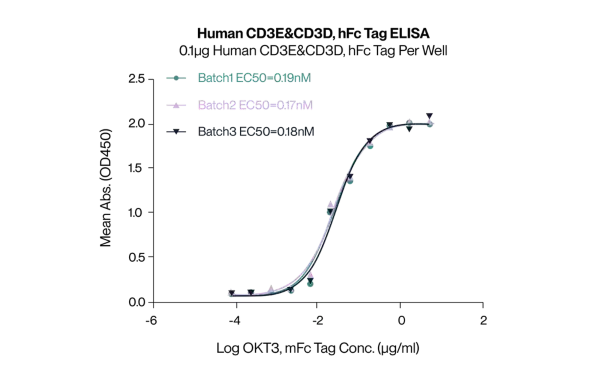
Immobilized Human CD3E/CD3G, hFc Tag, at 1μg/mL (100μL/well). Dose-response curve for anti-CD3E/CD3G antibody (OKT3, mFc Tag). The EC50s are 20.7, 21.0 and 20.ong/mL respectively as determined by ELISA.
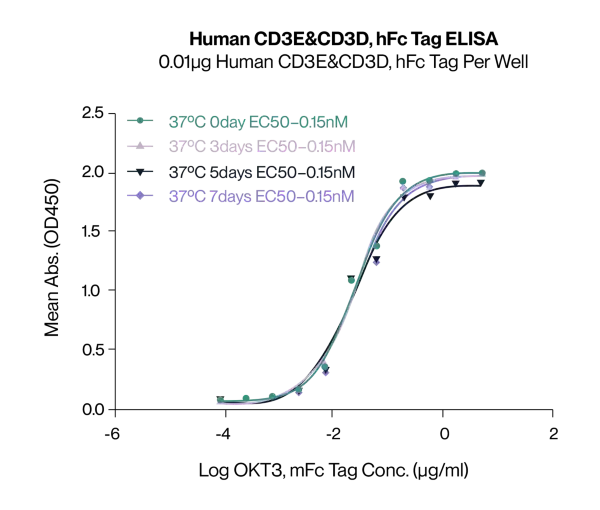
Temperature Stability
Immobilized Human CD3E/CD3G, hFc Tag, at 1μg/mL (100μL/well). Dose-response curve for anti-CD3E/CD3G antibody (OKT3, mFc Tag). The EC50s are 20.7, 19.5, 20.0 and 20.7ng/mL respectively, as determined by ELISA
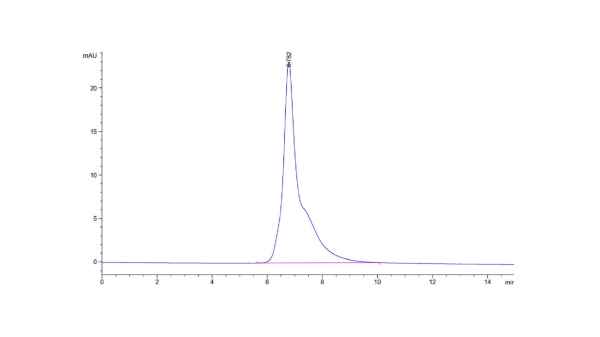
High Purity by SEC-HPLC
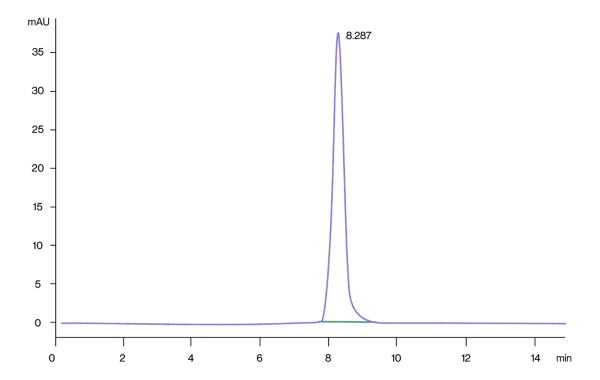
The purity of Cynomolgus CD3E is greater than 95% as determined by SEC-HPLC.
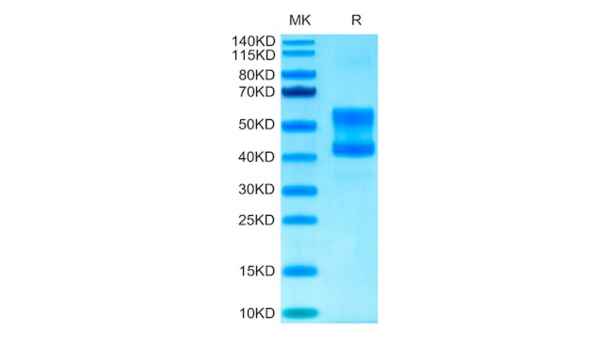
High Purity by Tris-Bis PAGE
Cynomolgus CD3E/CD3D on Tris-Bis PAGE under reduced conditions. The purity is greater than 95%.
Browse CD3 Proteins
Browse our popular CD3 proteins here or click below to view the full catalog selection:
More Products:
Background
FGFR (Fibroblast Growth Factor Receptors), generally includes four types of FGFR1-4 (also known as CD331-334). FGFR is a branch of the tyrosinase receptor family, which is a type I transmembrane protein usually functioning as a dimer. Among them, the extracellular domain contains three Ig-like regions D1 (IgI), D2 (IgII), and D3 (IgIII), of which D1 and the Acidic box form an autoinhibitory region, and D2 and D3 are responsible for ligand binding (D2 and cell surface Heparan sulfate is combined, D3 has two forms of IIIb and IIIc due to alternative splicing, and only one isoform of IIIc is currently found in FGFR4). According to the number of Ig-like domains, FGFR can be divided into two forms, one is α-type, which contains three regions of IgI, IgII, and IgIII; the other is β-type, which only contains IgII and IgIII.
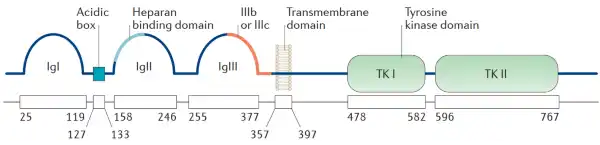
Figure 1. FGFR structure (Babina IS, et al., 2017)
The ligand of FGFR is FGF. There are 18 kinds of human FGFs that have been discovered. Except for FGF19, FGF21, and FGF23, which are endocrine, the rest are paracrine. Among them, FGF7 can only bind to type IIIb FGFR2. The combination of FGFR and FGF mediates the activation and transmission of signaling pathways such as RAS-RAF-MAPK, PI3K-AKT, JAK-STAT and PLCγ, and participates in important physiological functions such as cell growth, differentiation, migration, neovascularization, regulation of organ development and wound healing. FGFR gene mutations are commonly found in solid tumors such as lung cancer, liver cancer, intrahepatic cholangiocarcinoma, breast cancer, gastric cancer, uterine cancer, and bladder cancer, and there are differences in the types and frequencies of FGFR mutations in different cancer types.
Figure 2. FGF-FGFR-mediated signaling pathway (Mason I, 2007)
KACTUS has deeply analyzed the structural differences of FGFR family proteins and successfully prepared various types of FGFR recombinant proteins, covering α and β isoforms, as well as different forms such as IIIb and IIIc, supporting the differentiated research of FGFR-targeted drugs.
Product Performance Validation
Human FGFR2 beta (IIIb) Protein (FGR-HM1BB)
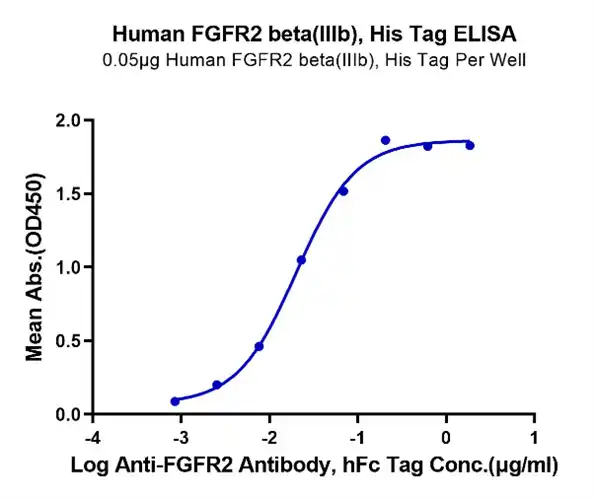
Figure 3. Immobilized Human FGFR2 beta(IIIb) at 0.5μg/ml on the plate. Dose response curve for Anti-FGFR2 Ab., hFc Tag with the EC50 of 20.2ng/ml determined by ELISA.
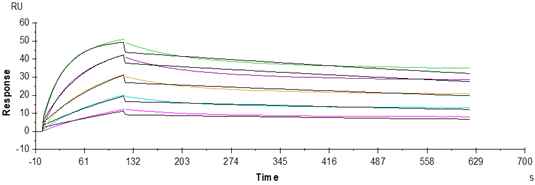
Figure 4. Anti-FGFR2 Ab., hFc Tag can bind Human FGFR2 beta IIIb, His Tag with an affinity constant of 1.98nM as determined in a SPR assay (Biacore T200).
Human FGFR2 alpha (IIIb) Protein (FGR-HM1BD)
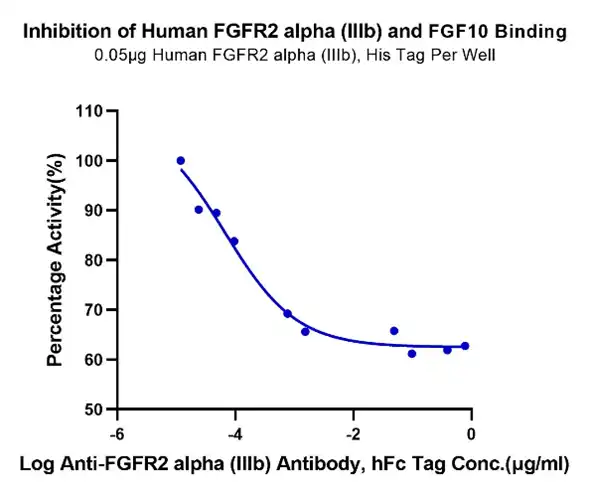
Figure 5. Serial dilutions of Anti-FGFR2 alpha (IIIb) Antibody were added into Human FGFR2 alpha (IIIb), His Tag : Biotinylated Human FGF10, No Tag binding reactioins.
Products
| Catalog No. | Description | Tag | Exact Sequence |
| KGF-HE101B | Biotinylated Human FGF-7/KGF Protein (Primary Amine Labeling) | N-His | Cys32-Thr194 |
| FGF-HM621B | Biotinylated Human FGF21 Protein | N-mFc-Avi | His29-Ser209 |
| FGF-HM4RAB | Biotinylated Human FGFR1 alpha (IIIc) Protein | C-His-Avi | Arg22-Glu374 |
| FGF-HM41CB | Biotinylated Human FGFR1 beta (IIIc) Protein | C-His-Avi | Lys158-Thr355 |
| FGF-HM4AB | Biotinylated Human FGFR2 alpha (IIIb) Protein | C-His-Avi | Arg22-Glu378 |
| FGR-HM4CDB | Biotinylated Human FGFR2 alpha (IIIc) Protein | C-His-Avi | Arg22-Glu377 |
| FGF-HM4BDB | Biotinylated Human FGFR2 beta (IIIb) Domain Protein | C-Avi | Pro253-Glu378 |
| FGR-HM4BBB | Biotinylated Human FGFR2 beta (IIIb) Protein | C-His-Avi | Arg152-Glu378 |
| FGR-HM4BCB | Biotinylated Human FGFR2 beta (IIIc) Protein | C-His-Avi | Arg152-Glu377 |
| FGF-HM43BB | Biotinylated Human FGFR3 alpha (IIIb) Protein | C-His-Avi | Glu23-Gly377 |
| FGF-HM43CB | Biotinylated Human FGFR3 alpha (IIIc) Protein | C-His-Avi | Glu23-Gly375 |
| FGF-HM4BBB | Biotinylated Human FGFR3 beta (IIIb) Protein | C-His-Avi | Asp127-Gly377 |
| FGF-HM4BCB | Biotinylated Human FGFR3 beta (IIIc) Protein | C-His-Avi | Asp127-Gly377 |
| FGF-HM4RBB | Biotinylated Human FGFR4 beta Protein | C-His-Avi | Pro152-Asp369 |
| FGF-HM4R4B | Biotinylated Human FGFR4 Protein | C-His-Avi | Leu22-Asp369 |
| FGF-MM43BB | Biotinylated Mouse FGFR3 alpha (IIIb) Protein (Primary Amine Labeling) | C-His | Pro22-Val349 |
| FGF-CM121 | Cynomolgus FGF21 Protein | C-His | His29-Ser209 |
| FGF-CM1BB | Cynomolgus FGFR2 beta (IIIb) Protein | C-His | Pro154-Lys368 |
| FGF-HM12T | Hamster FGF21-Protein | C-His | Arg29-Ser209 |
| KLB-HM101 | Human Beta Klotho Protein | C-His | Met30-Thr983 |
| FGF-HE002 | Human FGF basic (154aa) Protein | No Tag | Ala135-Ser288 |
| FGF-HE001 | Human FGF basic Protein | No Tag | Pro143-Ser288 |
| KGF-HE101 | Human FGF-7/KGF Protein | N-His | Cys32-Thr194 |
| FGF-HE010 | Human FGF10 Protein | No Tag | Gln38-Ser208 |
| FGF-HM121 | Human FGF21 Protein | N-His | His29-Ser209 |
| FGF-HM621 | Human FGF21 Protein | N-mFc-Avi | His29-Ser209 |
| FGF-HM4RA | Human FGFR1 alpha (IIIc) Protein | C-His-Avi | Arg22-Glu374 |
| FGF-HM41C | Human FGFR1 beta (IIIc) Protein | C-His-Avi | Lys158-Thr355 |
| FGF-HM2RA | Human FGFR2 alpha (IIIb) Protein | C-hFc | Arg22-Glu378 |
| FGR-HM1BD | Human FGFR2 alpha (IIIb) Protein | C-His | Arg22-Glu378 |
| FGR-HM2CD | Human FGFR2 alpha (IIIc) Protein | C-His | Arg22-Glu377 |
| FGF-HM0BD | Human FGFR2 beta (IIIb) Domain Protein | C-Avi | Pro253-Glu378 |
| FGF-HM2BD | Human FGFR2 beta (IIIb) Domain Protein | C-hFc | Pro253-Glu378 |
| FGF-HM12D | Human FGFR2 beta (IIIb) Protein | C-His | Pro154-Leu358 |
| FGR-HM1BB | Human FGFR2 beta (IIIb) Protein | C-His | Arg152-Glu378 |
| FGR-HM2BB | Human FGFR2 beta (IIIb) Protein | C-hFc | Arg152-Glu378 |
| FGR-HM1BC | Human FGFR2 beta (IIIc) Protein | C-His | Arg152-Glu377 |
| FGF-HM43B | Human FGFR3 alpha (IIIb) Protein | C-His-Avi | Glu23-Gly377 |
| FGF-HM43C | Human FGFR3 alpha (IIIc) Protein | C-His-Avi | Glu23-Gly375 |
| FGF-HM4BB | Human FGFR3 beta (IIIb) Protein | C-His-Avi | Asp127-Gly377 |
| FGF-HM4BC | Human FGFR3 beta (IIIc) Protein | C-His-Avi | Asp127-Gly375 |
| FGF-HM4RB | Human FGFR4 beta Protein | C-His-Avi | Pro152-Asp369 |
| FGF-HM2R4 | Human FGFR4 Protein | C-hFc | Leu22-Asp369 |
| FGF-HM4R4 | Human FGFR4 Protein | C-His-Avi | Leu22-Asp369 |
| FGF-MM121 | Mouse FGF21 Protein | N-His | His29-Ser210 |
| FGF-MM1BB | Mouse FGFR2 beta (IIIb) Protein | C-His | Pro39-Pro263 |
| FGF-MM43B | Mouse FGFR3 alpha (IIIb) Protein | C-His | Pro22-Val349 |
| FGF-CM1R4 | Rhesus macaque FGFR4 Protein | C-His | Leu22-Asp369 |
Background
Immune blockade therapy is becoming a new weapon in oncology due to the development of immune checkpoint proteins targeting in cancer, especially after the success of antibody drugs targeting programmed cell death-1 (PD-1), programmed cell death ligand-1 (PD-L1) and cytotoxic T-lymphocyte antigen-4 (CTLA-4). Although immune checkpoint blockade therapy often leads to a more durable response than chemo or targeted therapies in some cases, clinical data shows a limitation that in most cancers, the response rate is low (10-30%). Therefore, more associated studies focus on the mechanisms of non-responsiveness, as well as developing new targeting strategies using other immune checkpoint proteins.
Utilizing our featured SAMSTM platform, KACTUS has developed a series of immune checkpoint proteins with multiple pre-labels.
Background
FcR is the receptor of immunoglobulin (Ig), which causes corresponding cell-specific immune response by binding to the specific region of the Fc segment of Ig, acting as a bridge between humoral immunity and cellular immunity. Gene polymorphisms of FcR enable it to induce various intracellular biological effects, which are closely related to the occurrence and development of various immune-related diseases such as autoimmune diseases SLE and RA. Fc receptor proteins are one of the most important protein families in the immune system, mediating antibody-dependent ADCP, ADCC and CDC effects, and playing a key role in immune system activation and antibody function.
Currently the most widely used are mainly IgG receptors including FcRn and FcγR. FcyRs can be divided into three classes: FcyRI, FcyRII and FcyRIII. FcγRI has high affinity for IgG (10-9M) and is mainly expressed in monocyte-macrophages. FcγRII, FcγRIII and IgG have low affinity (10-6M) and are widely distributed. FcγRII is divided into 3 types: FcγRIIa, FcγRIIb and FcγRIIc according to the structure of the cytoplasmic region. It exists in B cells, macrophages, polymorphonuclear cells, platelets and monocytes, and FcγRIIb is the only inhibitory receptor. FcγRIII is present in granulocytes, NK cells, macrophages and activated monocytes. The combination of FcγR and antibody can activate immune cells and produce endocytosis, phagocytosis, ADCC and other functions.
Products
| Catalog No. | Description | Tag | Exact Sequence |
| FRN-MM101 | Mouse FcRn Protein | C-His | Ser22-Ser297(FCGRT) & Ile21-Met119(B2M) |
| FRN-HM101 | Human FcRn Protein | C-His | la24-Ser297(FCGRT) & Ile21-Met119(B2M) |
| CD3-HM123 | Human CD23/Fc epsilon RII Protein | N-His | Asp48-Ser321 |
| FRN-HM401B | Biotinylated Human FcRn Protein | C-His-Avi | Ala24-Ser297(FCGRT) & Ile21-Met119(B2M) |
| CD3-HM123B | Biotinylated Human CD23/Fc epsilon RII Protein | N-His-Avi | Asp48-Ser321 |
| FRN-RM101 | Rat FcRn Protein | C-His | Ala23-Ser298(FCGRT) & Ile21-Met119(B2M) |
| FCR-HM41BB | Biotinylated Human Fc gamma RIIIB/CD16b (NA1) Protein | C-His-Avi | Gly17-Ser200 |
| FGR-CM4R3B | Biotinylated Cynomolgus Fc gamma RIII/CD16 Protein | C-His-Avi | Gly17-Gln208 |
| FRN-MM401B | Biotinylated Mouse FcRn Protein | C-His-Avi | Ser22-Ser297(FCGRT) & Ile21-Met119(B2M) |
| IGG-RM001 | Rabbit IgG Protein | No Tag | Ser101-Lys323(T185A, N284S) |
| FCR-HM31 | Human Fc gamma RIIIA/CD16a (V176) Domain 2 Protein | C-mFc | Gly107-Thr189 |
| FGR-MM4R3B | Biotinylated Mouse Fc gamma RIII/CD16 Protein | C-His-Avi | Leu32-Thr215 |
| FRN-CM401B | Biotinylated Cynomolgus/Rhesus macaque FcRn Protein | C-His-Avi | Ala24-Ser297(FCGRT) & Ile21-Met119(B2M) |
| FCR-HM11B | Human Fc gamma RIIIB/CD16b (NA1) Protein | C-His | Gly17-Ser200(NA1) |
| MFC-MM001 | Mouse IgG1 Fc Protein (MFC-MM001) | No Tag | Val98-Lys324 |
| IGG-HM004 | Human IgG4 Fc Protein | No Tag | Glu99-Gly326 |
| IGG-HM001 | Human IgG1 Fc Protein | No Tag | Asp104-Lys330 |
| FRN-CM10 | Cynomolgus/Rhesus macaque FcRn Protein | C-His | Ala24-Ser297(FCGRT) & Ile21-Met119(B2M) |
| FRI-MM164 | Mouse Fc gamma RI/CD64 Protein | C-His | Glu25-Pro297 |
| FRI-HM464B | Biotinylated Human Fc gamma RI/CD64 Protein | C-His-Avi | Gln16-Pro288 |
| FRI-HM464 | Human Fc gamma RI/CD64 Protein | C-His-Avi | Gln16-Pro288 |
| FGR-MM1R3 | Mouse Fc gamma RIII/CD16 Protein | C-His | Leu32-Thr215 |
| FGR-CM1R3 | Cynomolgus Fc gamma RIII/CD16 Protein | C-His | Gly17-Gln208 |
| FER-MM201 | Mouse Fc Epsilon RI alpha/FCER1a Protein | C-hFc | Ala24-Gln204 |
| FER-HM201 | Human Fc epsilon RI alpha/FCER1a Protein | C-hFc | Val26-Gln205 |
| FER-HM101 | Human Fc epsilon RI alpha/FCER1a Protein | C-His | Val26-Gln205 |
| FCR-MM162 | Mouse Fc gamma RIV/CD16-2 Protein | C-His | Gly21-Gln203 |
| FCR-HM43AB | Biotinylated Human Fc gamma RIIIA/CD16a (V176) Protein | C-His-Avi | Gly17-Gln208(V176) |
| FCR-HM43A | Human Fc gamma RIIIA/CD16a (V176) Protein | C-His-Avi | Gly17-Gln208(V176) |
| FCR-HM42BB | Biotinylated Human Fc gamma RIIIB/CD16b (NA2) Protein | C-His-Avi | Gly17-Ser200(NA2) |
| FCR-HM42B | Human Fc gamma RIIIB/CD16b (NA2) Protein | C-His-Avi | Gly17-Ser200(NA2) |
| FCR-HM42AB | Biotinylated Human Fc gamma RIIA/CD32a (R167) Protein | C-His-Avi | Ala36-Ile218(R167) |
| FCR-HM42A | Human Fc gamma RIIA/CD32a (R167) Protein | C-His-Avi | Ala36-Ile218(R167) |
| CDB-MM101 | Mouse Fc gamma RIIB/CD32b Protein | C-His | Thr40-Arg217 |
| CDB-HM401B | Biotinylated Human Fc gamma RIIB/CD32b Protein | C-His-Avi | Ala46-Pro217 |
| CDB-HM401 | Human Fc gamma RIIB/CD32b Protein | C-His-Avi | Ala46-Pro217 |
| CDB-CM101 | Cynomolgus Fc gamma RIIB Protein | C-His | Ala46-Pro224 |
| CDA-HM432B | Biotinylated Human Fc gamma RIIA/CD32a (H167) Protein | C-His-Avi | Ala36-Ile218(H167) |
| CDA-HM432 | Human Fc gamma RIIA/CD32a (H167) Protein | C-His-Avi | Ala36-Ile218(H167) |
| CDA-HM416B | Biotinylated Human Fc gamma RIIIA/CD16a (F176) Protein | C-His-Avi | Gly17-Gln208(F176) |
| CDA-HM416 | Human Fc gamma RIIIA/CD16a (F176) Protein | C-His-Avi | Gly17-Gln208(F176) |
| CDA-CM132 | Cynomolgus Fc gamma RIIA/CD32a Protein | C-His | Gln28-Pro208 |
Background
CD47 is an immunoglobulin that is overexpressed on the surface of many types of cancer cells. CD47 forms a signaling complex with signal-regulatory protein α (SIRPα), enabling the escape of these cancer cells from macrophage-mediated phagocytosis. In recent years CD47 has been shown to be highly expressed by various types of solid tumors and to be associated with poor patient prognosis in various types of cancer.
A growing number of studies have since demonstrated that inhibiting the CD47-SIRPα signaling pathway promotes the adaptive immune response and enhances the phagocytosis of tumor cells by macrophages. Improved understanding in this field of research could lead to the development of novel and effective anti-tumor treatments that act through the inhibition of CD47 signaling in cancer cells.
Products
| Catalog No. | Description | Product Tag | Exact Sequence |
| SRP-HM40G | Human SIRP Gamma/CD172g Protein | C-His-Avi | Glu29-Pro360 |
| SRP-HM1V2 | Human SIRP alpha V2/CD172a Protein | C-His | Glu31-Arg369 |
| CD7-HM047 | Human CD47 Protein | No Tag | Gln19-Pro139 |
| CD7-HM547B | Biotinylated Human CD47 Protein | C-hFc-Avi | Gln19-Pro139 |
| CD7-RM247 | Rat CD47 Protein | C-hFc | Gln19-Lys140 |
| SRP-HM40BB | Biotinylated Human SIRP Beta/CD172b Protein | C-His-Avi | Glu30-Ala369 |
| CD7-CM147 | Cynomolgus/Rhesus macaque CD47 Protein | C-His | Gln19-Glu141 |
| SRP-HM4V8B | Biotinylated Human SIRP alpha V8 Protein | C-His-Avi | Glu31-Arg369(S44L, S50T, I52T, H54R, V57A) |
| SRP-MM172 | Mouse SIRP alpha/CD172a Protein | C-His | Lys32-Asn373 |
| SRP-HM572B | Biotinylated Human SIRP alpha/CD172a Protein | C-hFc-Avi | Glu31-Arg370 |
| SRP-HM4V8 | Human SIRP alpha V8 Protein | C-His-Avi | Glu31-Arg369(S44L, S50T, I52T, H54R, V57A) |
| SRP-HM4V6B | Biotinylated Human SIRP alpha V6 Protein | C-His-Avi | Glu31-Arg370(S105P) |
| SRP-HM4V6 | Human SIRP alpha V6 Protein | C-His-Avi | Glu31-Arg370(S105P) |
| SRP-HM4V5B | Biotinylated Human SIRP alpha V5 Protein | C-His-Avi | Glu31-Arg370(S42F) |
| SRP-HM4V5 | Human SIRP alpha V5 Protein | C-His-Avi | Glu31-Arg370(S42F) |
| SRP-HM4V4B | Biotinylated Human SIRP alpha V4 Protein | C-His-Avi | Glu31-Arg370(E33G, L44S, T50S, T52I, R54H) |
| SRP-HM4V4 | Human SIRP alpha V4 Protein | C-His-Avi | Glu31-Arg370(E33G, L44S, T50S, T52I, R54H) |
| SRP-HM4V3 | Human SIRP alpha V3 Protein | C-His-Avi | Glu31-Arg369(H54L) |
| SRP-HM4V3B | Biotinylated Human SIRP alpha V3 Protein | C-His-Avi | Glu31-Arg369(H54L) |
| SRP-HM4V2B | Biotinylated Human SIRP alpha V2/CD172a Protein | C-His-Avi | Glu31-Arg369 |
| SRP-HM4BLB | Biotinylated Human SIRP Beta 1 Isoform 3 Protein | C-His-Avi | Glu30-Leu371 |
| SRP-HM4BL | Human SIRP Beta 1 isoform 3 Protein | C-His-Avi | Glu30-Leu371 |
| SRP-HM40GB | Biotinylated Human SIRP gamma/CD172g Protein | C-His-Avi | Glu29-Pro360 |
| SRP-HM40B | Human SIRP Beta/CD172b Protein | C-His-Avi | Glu30-Ala369 |
| SRP-HM2V2 | Human SIRP alpha V2/CD172a Protein | C-hFc | Glu31-Arg369 |
| SRP-HM272 | Human SIRP alpha/CD172a Protein | C-hFc | Glu31-Arg370 |
| SRP-HM172 | Human SIRP alpha/CD172a Protein | C-His | Glu31-Arg370 |
| SRP-CM172 | Cynomolgus SIRP alpha/CD172a Protein | C-His | Glu31-Arg370 |
| CD7-MM247 | Mouse CD47 Protein | C-hFc | Gln19-Lys140 |
| CD7-MM147 | Mouse CD47 Protein | C-His | Gln19-Lys140 |
| CD7-HM447B | Biotinylated Human CD47 Protein | C-His-Avi | GIn19-Pro139 |
| CD7-HM247 | Human CD47 Protein | C-hFc | Gln19-Pro139 |
| CD7-HM147 | Human CD47 Protein | C-His | Gln19-Pro139 |
Transforming growth factor β (TGF-β) is a highly pleiotropic cytokine that plays an important role in wound healing, angiogenesis, immunoregulation and cancer. The cells of the immune system produce the TGF-β1 isoform, which exerts powerful anti-inflammatory functions and is a master regulator of the immune response.
Products
| Catalog No. | Description | Product Tag | Exact Sequence |
| GAT-RM401 | Rat GARP&Latent TGF Beta 1 Complex Protein | C-His-Avi | Ile18-Asn628(GARP) & Leu30-Arg278(Latent TGF Beta 1) |
| TGF-HM103B | Biotinylated Human Latent TGF beta 3 Protein (Primary Amine Labeling) | N-His | Leu24-Ser412 |
| LAP-HM4B1 | Human LAP (TGF beta 1) Protein | N-His | Leu30-Arg278(C33S) |
| TGF-HM103 | Human Latent TGF beta 3 Protein | N-His | Leu24-Ser412 |
| GAT-HM402 | Human GARP&Latent TGF Beta 2 Complex Protein | C-His-Avi | His20-Leu628(GARP) & Leu21-Ser414(Latent TGF Beta 2) |
| GAT-RM401B | Biotinylated Rat GARP&Latent TGF Beta 1 Complex Protein | C-His-Avi | Ile18-Asn628(GARP) & Leu30-Arg278(Latent TGF Beta 1) |
| TG2-HM00MB | Biotinylated Human Mature TGF beta 2 Protein | C-Avi | Ala303-Ser414 |
| TG3-HM00MB | Biotinylated Human Mature TGF beta 3 Protein | C-Avi | Ala301-Ser412 |
| TGF-HM6R1B | Biotinylated Human TGFBR1 Protein | C-mFc-Avi | Leu34-Glu125 |
| TGF-MM201 | Mouse TGF-alpha Protein | N-hFc | Val39-Ala88 |
| TGF-HM6R1 | Human TGFBR1 Protein | C-mFc-Avi | Leu34-Glu125 |
| TGF-HM5R2 | Human TGF-beta RII/TGFBR2 Protein | N-hFc | Ile24-Asp159 |
| TGF-HM3R2B | Biotinylated Human TGF-beta RII/TGFBR2 Protei | C-mFc-Avi | Ile24-Asp159 |
| TGF-HM3R2 | Human TGF-beta RII/TGFBR2 Protein | C-mFc-Avi | Ile24-Asp159 |
| TGF-HM201 | Human TGF-alpha Protein | N-hFc | Val40-Ala89 |
| TGF-HM102 | Human Latent TGF beta 2/TGFB2 Protein | N-His | Leu21-Ser414 |
| TG3-HM00M | Human Mature TGF beta 3 Protein | No Tag | Ala301-Ser412 |
| TG2-HM00M | Human Mature TGF beta 2 Protein | No Tag | Ala303-Ser414 |
| TG1-MM101 | Mouse Latent TGF beta 1/TGFB1 Protein | N-His | Leu30-Ser390 |
| TG1-HM401B | Biotinylated Human Latent TGF beta 1/TGFB1 Protein | N-His-Avi | Leu30-Ser390 (C33S) |
| TG1-HM401 | Human Latent TGF beta 1/TGFB1 Protein | N-His | Leu30-Ser390(C33S) |
| TG1-HM10MB | Biotinylated Human Mature TGF beta 1 Protein | C-Avi | Ala279-Ser390 |
| TG1-HM00M | Human Mature TGF beta 1 Protein | No Tag | Ala279-Ser390 |
| TG1-CM102 | Rhesus macaque Latent TGF beta 1/TGFB1 Protein | N-His | Leu30-Ser390(C33S) |
| LAP-HM4B1B | Biotinylated Human LAP (TGF beta 1) Protein | N-His-Avi | Leu30-Arg278(C33S) |
| GAT-MM401B | Biotinylated Mouse GARP&Latent TGF beta 1 Complex Protein | N-His-Avi | Ala18-Leu628(GARP) & Leu30-Ser390(Latent TGF beta 1) |
| GAT-MM101 | Mouse GARP&Latent TGF beta 1 Complex Protein | N-His | Ala18-Leu628(GARP) & Leu30-Ser390(Latent TGF beta 1) |
| GAT-HM405 | Human GARP (Y137H&S138G&G139N) &Latent TGF Beta 1 Complex Protein | C-His-Avi | His20-Leu628(GARP(Y137H&S138G&G139N)) & Leu30-Ser390(Latent TGF Beta 1) |
| GAT-HM401B | Biotinylated Human GARP&Latent TGF beta 1 Complex Protein | C-His-Avi | His20-Leu628(GARP) & Leu30-Ser390(Latent TGF beta 1) |
| GAT-HM401 | Human GARP&Latent TGF Beta 1 Complex Protein | C-His-Avi | His20-Leu628(GARP) & Leu30-Ser390(Latent TGF beta 1) |
| GAT-HM104 | Human GARP (G139N) &Latent TGF Beta 1 Complex Protein | C-His-Avi | His20-Leu628(GARP(G139N)) & Leu30-Ser390(Latent TGF beta 1) |
| GAT-HM103 | Human GARP (S138G) &Latent TGF Beta 1 Complex Protein | C-His-Avi | His20-Leu628(GARP(S138G)) & Leu30-Ser390(Latent TGF Beta 1) |
| GAT-HM102 | Human GARP (Y137H) &Latent TGF Beta 1 Complex Protein | C-His-Avi | His20-Leu628(GARP(Y137H)) & Leu30-Ser390(Latent TGF Beta 1) |
| GAT-CM401B | Biotinylated Cynomolgus GARP&Latent TGF beta 1 Complex Protein | C-His-Avi | Ala18-Leu628(GARP) & Leu30-Ser390(Latent TGF beta 1) |
| GAT-CM401 | Cynomolgus GARP&Latent TGF beta 1 Complex Protein | C-His-Avi | Ala18-Leu628(GARP) & Leu30-Ser390(Latent TGF beta 1) |
