Chemiluminescent Western blotting
 Chemiluminescent Western blotting is a highly sensitive protein detection method. The broad dynamic range allows analyte detection over a wide range of protein concentration. The linear response of reporter enzymes combined with enhanced chemiluminescent (ECL) substrates makes the detection method suitable for quantitative assays.
Chemiluminescent Western blotting is a highly sensitive protein detection method. The broad dynamic range allows analyte detection over a wide range of protein concentration. The linear response of reporter enzymes combined with enhanced chemiluminescent (ECL) substrates makes the detection method suitable for quantitative assays.
Reporter enzyme conjugated secondary antibodies can be used for chemiluminescent detection when combined with an appropriate chemiluminescent substrate. As described in figure 1, a reporter enzyme catalyzes the conversion of a chemiluminescent substrates to a light emitting product. The light is then captured either using a charge coupled device (CCD) digital camera or by X-ray film, identifying the protein of interest.
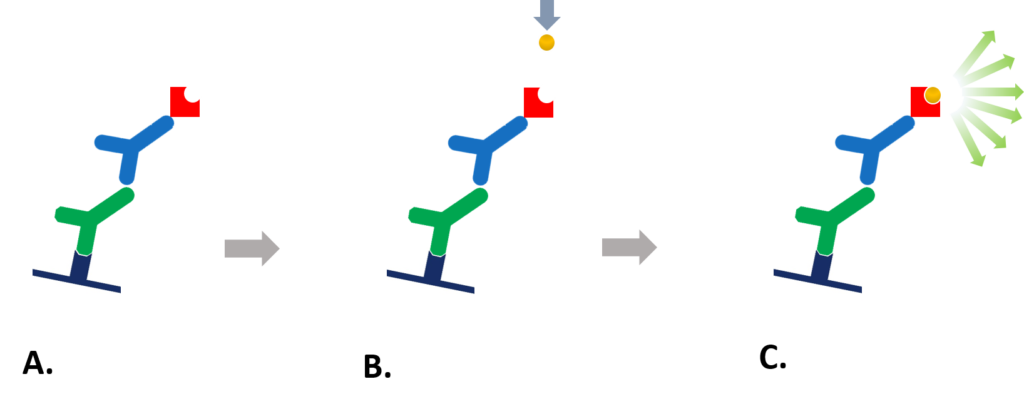
Figure 1: Chemiluminescent detection. A. Enzyme-conjugated secondary antibody is bound to primary antibody at protein of interest. B. Chemiluminescent substrate is added to antibody-antigen complex. C. The reporter enzyme catalyzes the substrate to a light emitting product, and the signal can be detected by (CCD) digital imager or x-ray film.
Signal stability
The signal output from the analyte is transient, only produced during the enzyme-substrate reaction. The signal will decay upon exhaustion of the substrate, though the enzyme will remain active. In some cases the substrate can last up to 24 hrs, but in situations with high concentrations of analyte, signal output may be as brief as a few minutes. Analyte signal can be refreshed by adding new substrate if required.
To optimize signal to background capture and to avoid signal saturation, multiple exposures may be required. If using X Ray film, development may require a 24 hr exposure in cases of low antigen or poor primary antibody affinity, and may use a number of films during the optimization process. Digital imaging simplifies exposure optimization with signal capture between 5 seconds and 5 minutes, and images can be generated with minimal waste of resources or time.
Quantitation
The linear nature of the ECL substrate catalysis by the reporter enzyme makes it ideal for quantitative Western blotting. Image J and Image Quant are among a number of image analysis software available to evaluate the density (densitometry) of analyte bands relative to control bands, and thus derive the relative quantity of the analyte.
The blot must be set up so that detection is not at the saturation limit, determined by titration of experimental reagents. Loading controls must be included, and background signal must be compensated for. It should be noted that if very accurate quantification is required, an ELISA which uses a standard curve may be a more applicable.
Sensitivity
Chemiluminescent detection is very sensitive, and detection of picogram amounts is routinely reported. With optimization, femtogram amounts of analyte may be detectable. Chemiluminescent substrates with varying sensitivity are available, and choice of substrate may depend on analyte abundance.
Multiplexing – Detection of multiple analytes on the same blot
Detection of multiple proteins on the same blot may be useful in testing for protein expression when the protein has multiple tags, for example His6 for purification and a more sensitive FLAG for detection. If the first primary doesn’t detect the tag in the sample due to truncation, proteolysis or faulty primary, the researcher can probe for a second tag to confirm expression.
Chemiluminescent Western blots cannot be multiplexed, however they can be stripped using a stripping buffer and re-probed with a further pair of antibodies. The stripping buffer is usually harsh and may alter the signal achieved with the second primary, so stripped blots are not recommended for quantitative analysis. Fluorescent Western blotting offers a much more reliable method for detection of multiple antigens – Learn more about Fluorescent Western blotting.
Horseradish peroxidase conjugates
The reporter enzyme horseradish peroxidase (HRP) is a popular secondary antibody conjugate. Combined with a peroxide buffer, HRP oxidizes the chemiluminescent substrate luminol to its excited state product, 3-aminophthalate. The product emits light at 425 nm as it decays to its ground state, and this signal can be captured by x-ray film or CCD camera.
Alkaline phosphatase conjugates
Alkaline phosphatase can be used for chemiluminescent detection with the substrate Disodium-chloro-5-(4-methoxyspiro{1,2-dioxetane-3,2’-(5’-chloro)tricyclodecan}-4-yl)-1-phenyl phosphate), which is dephosphorylated by AP to yield metastable dioxetane phenolate anion that emits light at 466 nm (Schaap et al 1989) . The emitted light is stable for up to 24 hours, allowing for multiple exposures to X-Ray films.
Horseradish peroxidase and alkaline phosphatase conjugates are sensitive reagents suitable for all immunotechniques.