How to Choose Membrane Protein Isolation Kits
Plasma Membrane Isolation Kit
Plasma membrane (PM) proteins play a critical role in a variety of physiological and pathological processes. Signal transduction, molecular transport and cell-cell interactions are all mediated by PM proteins. PM proteins include a variety of important proteins such as neurotransmitter receptors, G-proteins, carriers, voltage-gated ion channels, CD antigens and many drug targets. The detection, characterization, and intracellular trafficking of PM proteins are, therefore, essential for understanding of biological systems.
Isolation/purification is usually the first step for characterization and profiling of PM proteins. However, it has been proven to be particularly challenging because of their low abundance and the nature of inter-connectivity of intracellular membrane systems. PM proteins are traditionally isolated by sucrose density ultra-centrifugation [1,2]. The protocol is tedious and time consuming and takes hours to even days to complete. In recent years, more and more publications cited commercial kits for PM protein isolation and characterization due to their ease of use and speed. However, different kits employee different mechanisms of action. The efficacy of the kit varies significantly depending upon specific downstream applications. Due to the availability of multiple PM protein isolation/purification kits in the market, it is, sometimes, difficult or even confusing for selection of a proper kit for a particular research project. In this context, we attempt to summarize pro and cons of some of the commonly used membrane protein isolation kits and provide a general guide for selection of commercial membrane protein isolation kits.
Generally speaking, all commercially available membrane protein isolation kits can be classified into four basic categories according to their mechanisms of action or principles of isolation.
1. Phase extraction. Mem-PER™ Plus Membrane Protein Extraction Kit (Thermo Fisher), Mem-PER® Eukaryotic Membrane Protein Extraction Reagent Kit (Thermo Fisher) and ProteoExtract™ Native Membrane Protein Extraction Kit (Millipore-Sigma) are typical kits included in this class. Cells/tissues are first lysed by lysis buffer, soluble proteins and insoluble fractions are separated by centrifugation. Membrane proteins are further extracted from insoluble fraction by an extraction buffer based on hydrophobicity of membrane proteins. These kits are relative simple and rapid (about 1h). The protocol generates two distinctive portions: cytosolic fraction and membrane protein fraction however it is not clear from the protocol whether a detergent is used for cell lysis. Membrane proteins extracted are derived from all membrane systems such as mitochondria, ER, Golgi and nuclei. What extracted by these kits are actually total membrane proteins. It can be anticipated that the membrane protein extraction may or may not be complete for certain samples especially for those proteins that transverse bio-membrane multiple times. It is not clear if membrane associated proteins remain bound and intact in the extracted membrane proteins.
2. Cell surface labeling. Pierce cell surface protein isolation kit (Thermo Fisher) and Qproteomic Plasma membrane kit (Qiagen) belong to this class. A typical experiment using pierce cell surface protein isolation kit involves labeling of cell surface proteins by sulfo-NHS-biotin. After labeling, cells are lysed and the cell lysate is applied to an avidin-conjugated solid phase. Biotinylated plasma membrane proteins are eluted using a denaturing elution buffer. In theory, this approach should produce highly purified plasma membrane proteins. However, in reality, This method suffers many inherited disadvantages. For example, not every protein on the cell surface will be labeled. The protein profile of a given cell culture may change with cultured time and conditions. Steric hindrance and lack of primary amines may interfere with protein labeling resulting in inconsistent results. The use of denatured elution solution also limits the application of isolated proteins.
3. Phase partitioning. A typical example of this class is the Plasma Membrane Protein Extraction Kit from Biovision and Abcam. The mechanisms of action is similar to an earlier published aqueous two phase partitioning method [3]. The protocol takes the advantage of differential partitioning of plasma membrane in the upper phase and other membranes (such as ER, Golgi and mitochondria) in lower phase for enrichment and isolation of plasma membrane proteins. Cultured cells/tissue samples are first homogenized using a Dounce homogenizer. The lysed samples are subject to multiple extraction and centrifugations resulting in total membrane protein, cytosolic and plasma membrane protein fractions. The pros of the method are to be able to isolate detergent-free plasma membrane proteins and the protocol is relatively sample and rapid (about 1 -1.5 h). However, large cell numbers are required (50-100 millions/sample) and the yield is relatively low (1-100ug/sample). It was claimed by the manufacturer that the purity of plasma membrane protein is over 90% but no supporting data are shown.
4. Spin column-based subcellular fractionation. This is a next generation plasma membrane protein isolation technology from Invent biotechnologies featuring a simple and rapid method for subcellular fractionation without using a Dounce homogenizer. Cells/tissues are passed through a specialized filter cartridge. Plasma membranes of the cells are ruptured during the process and intact nuclei, organelles, plasma membrane, and cytosolic proteins are released into a suspension which is further separated into five fractions: total membrane, plasma membrane, cytosolic, organellar, and nucleus fractions. Due to the use of the filter cartridge and a unique buffer system, high yield of native plasma membrane protein can be obtained in less than one hour with minimum cross-contaminations. The native plasma membrane protein isolated can be used for any downstream experiments. Unlike all other kits described above, the spin column-based plasma membrane isolation kit can also be used for plant PM protein isolation. This kit is becoming more and more popular as evidenced by the data from selected publications below.
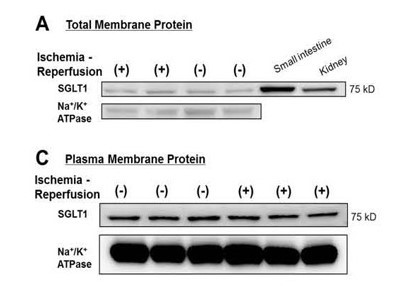
The selection of a particular membrane protein isolation kit mainly depends on specific downstream applications. Some PM isolation kits have much broader applications than others. For instance, detergent-free native PM proteins can be used for almost all possible downstream applications, while those isolated by Pierce cell surface protein isolation kit is only recommended for Western blotting. Following examples illustrate what is expected from a good subcellular fractionation kit. Many PM proteins are present in relatively low concentration. Sometimes it is difficult to detect and quantify membrane proteins without isolation and enrichment. Following data [4] clearly demonstrate the effect of PM protein enrichment on detectability of SGLT1 from human heart samples. The SGLT and plasma membrane marker protein Na+/K+ ATPase signals are significantly enhanced in plasma membrane fraction as compared to total membrane fraction.
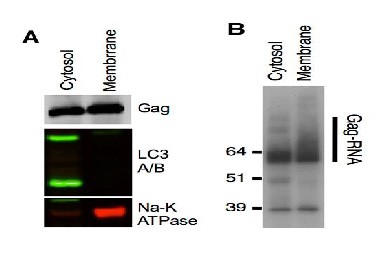
Kutluay et al. [5] studied the changes in Gag-RNA binding to viral RNA during HIV-1 virion assembly. One of the key experiment is to demonstrate different form of Gag-RNA adducts present in different subcellular locations. 4SU-fed 293T cells were transfected with HIV-1 proviral plasmid and fractionated by spin column-based plasma membrane protein isolation kit. The cytosolic and plasma membrane fractions were subjected to Western blotting and immunoprecipitation. The results indicate that the cytosolic Gag protein is primarily monomeric, while Gag protein on plasma membrane is multimerized (B). This conclusion heavily depends on clear separation of cytosolic and plasma membrane fractions (A). if significant cross-contamination is present, the conclusion would be questionable.
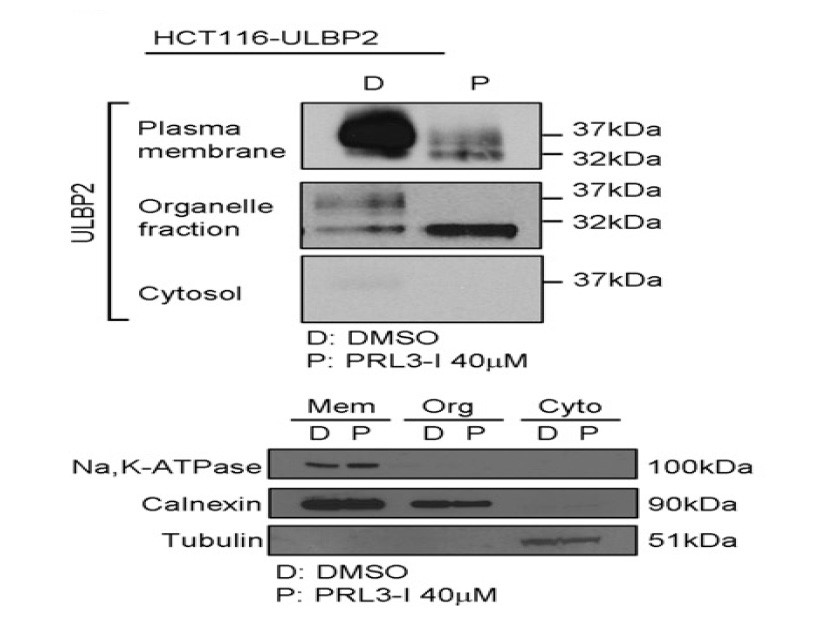
Studies of Intracellular target protein distribution and target protein trafficking under different experimental conditions and different treatment of experimental groups are important for biomedical research. The target protein can be tracked by subcellular fractionation followed by a specific detection method. Leung et al. [6] studied the effect of PRL-3 on ULBP2 protein trafficking by cell fractionation. Human colon cancer cell line HCT 116 was treated with 40 um RPL-3 and subjected to subcellular fractionation using a spin column-based plasma membrane isolation kit. The results showed a clear separation of PM, organelle and cytosolic fractions. Treatment of the cell with the chemical results in translocation of plasma membrane to organelle fraction. Again this conclusion is based on clear separation of difference subcellular fractions.
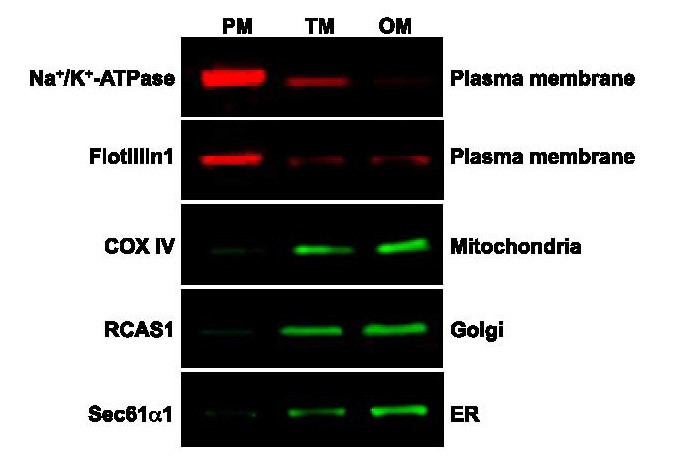
As demonstrated by Jose et al. [7], Spin-column PM isolation kit (SM-005, Invent Biotechnologies) can fractionate cultured endothelial cells into different subcellular fractions and most important of all, the isolated PM protein shows minimum/or no cross contamination from organelles such as mitochondria, ER and Golgi.
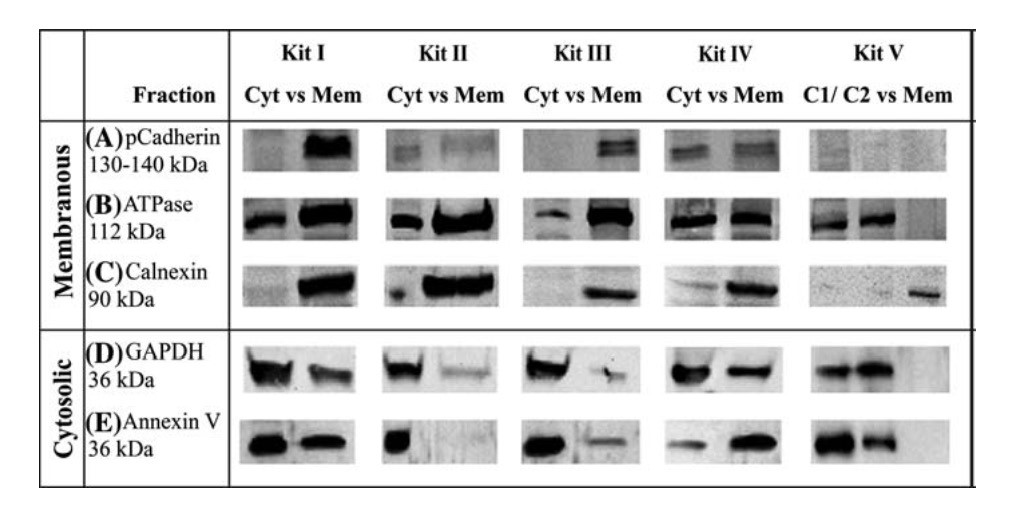
Degree of cross-contamination is obviously one of the most important factors for selection of a membrane protein isolation kit. The performance of commercial kits differs significantly with this criterion. Bunger et al. [8] compared five commercial membrane protein isolation kits for cross-contamination between cytosolic (Cyt) and plasma membrane (Mem) fractions and found that all kits tested show obvious cross-contamination for two commonly used markers (ATPase and GAPDH). The performance of the kits varies significantly when cadherin is used for a PM marker. It is highly recommended to choose a membrane protein isolation kit by referencing with high impact factor publications.
As a summary, membrane protein isolation kits are valuable tools for researchers. Compared to traditional methods, a good commercial kit can save time. increase efficiency and speed up discovery process. The selection of a particular kit should be mainly based on the performance and specific downstream experiments. Sample size required, sometimes, is an important factor to be considered especially when available cell number is a limiting factor. Under ideal conditions, a good commercial kit should provide researcher with expected results without significant trouble shooting and optimization. Another criterion to be considered is to determine if the kit can give researchers a competitive edge in terms of ease of use, speed, performance and cost.
References:
1. Neville, D. M, (1960) The isolation of a cell membrane fraction from rat liver. J. Biophy. Biochem. Cyto. 8: 413-421.
2. Touster, O. et al. (1970) Isolation of liver plasma membranes. J. Cell Bio. 47:604-618.
3. Yoshida, S. et al. (1983) Partitioning of membrane particles in aqueous two-polymer phase system and its practical use for purification of plasma membranes from plants.Plant physiol. 72:105-114.
4. Kashiwagi Y. et al. (2015) Expression of SGLT1 in Human Hearts and Impairment of Cardiac Glucose Uptake by Phlorizin during Ischemia- Reperfusion Injury in Mice. PLoS ONE 10(6):e0130605.
5. Kutluay, S., et al. (2014). Global Changes in the RNA Binding Specificity of HIV-1 Gag Regulate Virion Genesis, Cell (2014), https://dx.doi.org/10.1016/j.cell.2014.09.057
6. Leung W-H. et al. (2015). PRL-3 Mediates the protein Maturation of ULBP2 by regulating the tyrosine phosphorylation of HSP60.The Journal of Immunology.doi:10.4049/jimmunol.1400817.
7. Vazquez-Medina J.P. et al. (2016). The phospholipase A2 activity of peroxiredoxin 6 modulates NADPH oxidase 2 activation vialysophosphatidic acid receptor signaling in the pulmonary endothelium and alveolar macrophages. The FASEB Journal. doi: 10.1096/fj.201500146R
8. Bunger, S. et al. (2009) Comparison of five commercial extraction kits for subsequent membrane protein profiling. Cytotechnology. 61:153-159.