SALIMETRICS COVID-19
Research Solutions for Coronavirus
Serological surveillance research tools
The Salimetrics® SARS-CoV-2 N-protein Salivary IgG ELISA kit is an enzyme-linked immunoassay specifically designed and validated for the qualitative measurement of human IgG specific to the SARS-CoV-2 Nucleocapsid protein (N-protein) in oral fluid which can be utilized in research and surveillance testing. This assay kit is not intended for diagnostic use. Research in saliva has identified that SARS-CoV-2 antibody testing could serve as a sensitive and specific serum alternative for large scale immune-surveillance studies. For this assay, the N protein was chosen to maximize the likelihood of antibody detection, since it is the most immunodominant protein in the coronavirus family. Qualitative cutoff values are determined for each run based on controls provided in the kit and serostatus determined from values obtained by oral fluid testing. When used in conjunction with Total IgG, researchers can maintain sample quality assurance and determine a robust inclusion criteria when using oral fluid as an alternative sample type for serological studies.
Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) is the RNA virus strain that causes COVID-19, the disease first identified in 2019. In addition to virus exposure, there is an urgent need to better understand the levels and duration of protective immunity for public health considerations. Research in saliva has identified that SARS-CoV-2 antibody testing could serve as a sensitive and specific serum alternative for large scale immune-surveillance studies (1). Importantly, IgG in saliva is derived from serum, so specificity and reactivity of salivary IgG directly reflects serum IgG reactivity, making oral fluid an attractive alternative sample to blood for serological studies (2, 3).
Current research has shown that antibody levels decrease in the serum of COVID-19 patients over time (4) and vary depending on disease severity. In fact, a higher number of asymptomatic participants become seronegative at 60 days indicating a true decline over a 2-month period rather than an artifact of assay performance. Due to this, the current assay has been benchmarked to a serum specific anti-N protein assay instead of PCR assay results.
TECHNICAL SUMMARY
| TESTS PER KIT | |
| Singlet: | 76 |
| Duplicate: | 38 |
| TARGET ANALYTE |
| Salivary SARS-CoV-2 (N) IgG |
| TECHNICAL DOCUMENTATION |
| Stop Solution SDS |
SALIVARY COVID-19 (SARS-COV-2, N) IGG ELISA KIT OVERVIEW
INTENDED USE
The Salimetrics® SARS-CoV-2 N-protein Salivary IgG ELISA kit is an enzyme-linked immunoassay specifically designed and validated for the qualitative measurement of human IgG specific to the SARS-CoV-2 Nucleocapsid protein (N-protein) in oral fluid.
It is intended for surveillance testing and not for diagnostic use. This assay kit was optimized and validated for performance in human oral fluid and has not been validated for other human sample types, such as human serum or plasma.
Surveillance testing for Antibodies can be used to determine if an individual may have been exposed to and infected with a virus, and also can be used to understand how many people in a population have antibodies (known as “surveillance tests,” or sero-surveys). (From the FDA guidance document (https://www.fda.gov/media/137599/download))
• When used for surveillance, the results can help determine how widely the virus has spread in communities and how far the pandemic has progressed. Results from tests used for surveillance only are generally not shared with individual patients and are critical for understanding the extent of and risk factors associated with infection.
• Testing individuals may help identify who has developed antibodies against SARS-CoV-2. The results of ongoing research are needed before it is known whether these antibodies are associated with protection from future infection. Current results can help inform who may qualify to donate blood that can be used to manufacture convalescent plasma.
Please read the complete kit insert before performing this assay. Failure to follow kit procedure and recommendations for saliva collection and sample handling may result in unreliable values.
INTRODUCTION
The novel severe acute respiratory syndrome coronavirus 2 (SARS-Cov-2) was initially identified in the Winter of 2019 with an outbreak designation soon after at the end of the year (https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7190497/). Since that time, the virus rapidly spready to achieve global pandemic status and quickly resulted in widespread containment and confinement public health measures in most countries throughout the world. These included shutdowns of schools, businesses, travel restrictions and physical/social distancing that had varying degrees of success resulting in a continued global circulation of the virus. With the current infection rates in the US and resurgence in many countries, elimination of the virus is no longer possible and more long-term strategies to minimize the health and economic impact will be needed for years to come.
In addition to virus exposure, there is an urgent need to better understand the levels and duration of protective immunity for public health considerations. Adding serology will help in this effort and many serum assays are available in many formats, however for larger scale efforts to measure disease incidence, there is a need for an at home painless collected sample that simultaneously minimizes exposure risk of patient and healthcare worker that can be met with an oral fluid offering. Our assay will meet the technical need for high sensitivity and specificity performance in saliva for central testing at scale. The main source of salivary IgG antibodies is the serum, so it is not surprising that salivary IgG antibodies directly reflect the specificity and activity of those found in serum (2, 3). Oral fluid is thus an easily accessible surrogate to serum or plasma in this regard and enables salivary serology studies, surveillance tests or sero-surveys (1). Testing can help determine who has developed antibodies against SARS-CoV-2. Importantly, we have determined that human IgG withstands conditions commonly used for viral heat inactivation (60 or 65 C for 30 min and 95 C for 5 min). The main utility of antibody tests for SAR- CoV-2 supported by the WHO, CDC, FDA and AMA are surveillance studies and the Salimetrics N-protein IgG ELISA kit meets this need.
Antibody levels decrease in the serum of COVID19 patients over time (4, 5) and vary depending on disease severity (6). In fact a higher number of asymptomatic participants become seronegative at 60 days indicating a true decline over a 2-month period rather than an artifact of assay performance (4, 5). Therefore, our assay is benchmarked on validated commercial serum kit performance instead of molecular tests. Maximizing the likelihood of antibody detection, N protein was chosen as an advantage since it represents the most immunodominant protein in the coronavirus family.
Antibody levels decrease in the serum of COVID19 patients over time and vary depending on disease severity. In fact a higher number of asymptomatic participants become seronegative at 60 days indicating a true decline over a 2-month period rather than an artifact of assay performance. Therefore, our assay is benchmarked on validated commercial serum kit performance instead of molecular tests. Maximizing the likelihood of antibody detection, N protein was chosen as an advantage since it represents the most immunodominant protein in the coronavirus family.
SALIVARY COVID-19 (SARS-CoV-2) IgG ASSAY PRINCIPLE
This is a salivary serological ELISA kit where viral antigen is coated on microtiter plates and human antibodies in test samples are detected using an Anti-Human IgG detection antibody linked to horseradish peroxidase (HRP). After each incubation, unbound components are washed away. Anti-Human IgG HRP Enzyme Conjugate is then added and the levels measured by the reaction of the HRP enzyme to the substrate tetramethylbenzidine (TMB). This reaction produces a blue color. A yellow color is formed after stopping the reaction with an acidic solution. The optical density is read on a standard plate reader at 450 nm. The total amount of anti-IgG HRP Enzyme Conjugate detected is proportional to the amount of anti-SARS CoV2 IgG present in the sample. Qualitative cutoff values are determined for each run based on controls provided in the kit and serostatus determined from values obtained by oral fluid testing. The cutoff OD is used to divide sample OD values to produce signal/cutoff ratio in a simple calculation with values above 1.1 positive and below 0.8 negative. Samples reading between these values are considered borderline and a second test is recommended for these samples or a second collection.
REFERENCES & SALIVARY COVID-19 RESEARCH
- Randad, PR., et al. (2020). COVID-19 serology at population scale: SARS-CoV-2-specific antibody responses in saliva. medRxiv.
- Hettegger, P., et al. (2019). High similarity of IgG antibody profiles in blood and saliva opens opportunities for saliva based serology. PloS one. 2019;14(6):e0218456.
- Heaney, JLJ., et al. (2018). The utility of saliva for the assessment of anti-pneumococcal antibodies: investigation of saliva as a marker of antibody status in serum Biomarkers. 23(2):115-22.
- Patel, M., et al. (2020). Change in Antibodies to SARS-CoV-2 Over 60 Days Among Health Care Personnel in Nashville, Tennessee. JAMA.
- Vabret N. (2020). Antibody responses to SARS-CoV-2 short-lived. Nat Rev Immunol. 20(9):519.
- Long QX., et al. (2020). Antibody responses to SARS-CoV-2 in patients with COVID-19. Nat Med. 26(6):845-8.
With a 96.5% sensitivity and 98.8% specificity, the Salimetrics SARS-CoV-2 IgG Antibody Panel, 3-Plex has been validated to qualitatively detect antibodies to SARS-CoV-2 Nucleocapsid, Spike 1 and Spike 1 Receptor Binding Domain (RBD) in the same saliva (or serum) sample. This IgG multiplex assay utilized by Salimetrics is based on the same SARS-CoV-2 (IgG) Antibody Test that was implemented during COVID-19 vaccine clinical trials, adapted and fit-for-purpose validated for the detection of antibodies in saliva samples for research use. The SARS-CoV-2 IgG Antibody Panel can also effectively track the antibody response to vaccine or natural infection over time and provide relative levels by including a reference standard.
While the SARS-CoV-2 IgG Antibody Panel requires sending samples to Salimetrics, our project managers are available to assist in proper packaging and handling of saliva samples to be received at the Salimetrics Laboratory. Volume discounts are also available, please contact us for more information.
Technical Summary
| PANEL SUMMARY | |
| Optimum Collection Volume: | 100 μL |
| Total Number of Samples Required: | 1 |
| Methodology: | ECL |
| TARGET SARS-COV-2 ANTIBODIES |
| SARS-CoV-2 Spike Protein (S1) |
| SARS-CoV-2 Nucleocapsid Protein (N) |
| SARS-CoV-2 Receptor Binding Domain (RBD) |
| ASSAY PERFORMANCE* | |
| Sensitivity: | 96.5% |
| Specificity: | 98.8% |
| PREDICTIVE VALUE (AT 5% PREVALANCE)* | |
| PPV: | 81% |
| NPV: | 99.8% |
SALIMETRICS ASSAY #1-4502
The Salimetrics Salivary Total Human IgG (Immunoglobulin-G) Enzyme Immunoassay Kit was specifically designed to standardize the detection of human Immunoglobulin G in saliva samples for research and biomedical laboratories. Using a small sample volume, this assay kit has an extended range which spans the expected IgG levels found in human saliva. The average inter- and intra-assay precision coefficients of variation are low with no deleterious matrix effects often found in saliva which are characterized through dilution- and spike-recovery validation procedures. This IgG assay kit has also been formatted to minimize cross reactivity for related immunoglobulins.
Immunoglobulin G (IgG) is a key component of the humoral immune system for host immune-defense against pathogenic viruses and microbes. There are four subclasses of IgG (IgG1, IgG2, IgG3 and IgG4). Collectively, total IgG refers to all IgG subclasses, which have varying unique reactivities, present at a given time. In most cases an immune response includes a mixture of all four subclasses and this assay has been designed to recognize detect them all. IgG levels in saliva are generally in the microgram per milliliter range, while in blood they are much higher, in the milligram per milliliter range (1).
TECHNICAL SUMMARY
| ASSAY PROTOCOL |
| Download |
| SPECIFICATIONS | |
| Catalogue#: | 1-4502 |
| Regulatory Status: | RUO |
| Format: | 96-well plate |
| Assay Time: | ~ 4.5 hrs |
| Sample Volume/Test: | 10 µL |
| Sensitivity: | 0.94 µg/mL |
| Assay Range: | 0.3125 – 20 ng/mL |
| Storage Requirements: | 2-8°C |
| TESTS PER KIT | |
| Singlet: | 76 |
| Duplicate: | 38 |
| TARGET ANALYTE |
| Salivary IgG |
| TECHNICAL DOCUMENTATION |
| Stop Solution SDS |
SALIVARY IGG ASSAY KIT OVERVIEW
INTENDED USE
The Salimetrics® Salivary Human Total IgG ELISA Kit is an enzyme-linked immunoassay specifically designed and validated for the quantitative measurement of human total IgG in oral fluid. It is not intended for diagnostic use. This assay kit was optimized for human salivary research and has not been validated for other human sample types, such as serum or plasma or samples from other species.
INTRODUCTION
High titers of pathogen specific IgG are a measure of protective immunity against pathogens and are generated by an immune response to either a prior infection event or immunization (2). Most IgG in saliva originates from the serum entering into saliva passively through the gingival crevicular epithelium, however some is produced locally in the salivary glands or gingiva (3). Importantly, the reactivity of salivary IgG mirrors that of serum IgG, so oral fluid is an attractive alternative sample to blood for serological studies where antibody levels indicate an individual’s immune status to a pathogen of interest (4, 5). As an alternative to blood, oral fluid enables advantages like home collection or when sampling populations where blood draws are a challenge, for instance in small children or the elderly (6).
One important application for the measurement of total IgG in oral fluid is to qualify samples as having sufficient antibody levels to enable valid serological studies (6). In this case, since oral fluid may vary in the amount of antibody present at the time of sampling, to definitively determine if an individual shows negative reactivity, the sample must be tested for total IgG levels to assure there is adequate total antibody present in the test. Cutoff levels may be established to determine if a sample has adequate IgG as well as to help interpret the relative degree of positivity in samples that show reactivity.
In addition, it has been recently reported that levels of total IgG in oral fluids is highly correlated with proinflammatory cytokine levels, suggesting that, in certain applications, total salivary IgG could be used as a surrogate, and inexpensive marker, to index oral inflammation (7). In this case, total IgG in saliva may be a useful covariate in statistical analyses to control for the confounding effects of poor oral health. This might be very important if the study participants are at high risk for oral health problems.
SALIVARY HUMAN TOTAL IgG ASSAY PRINCIPLE
This is an indirect sandwich ELISA kit. A “sandwich” is formed when the pre-coated capture Anti-Human IgG antibody present on the plate binds IgG in standards & samples, which is then bound by the Anti-Human IgG detection antibody linked to horseradish peroxidase. After each incubation, unbound components are washed away. Bound Anti-Human IgG Antibody Enzyme Conjugate is then added and the levels measured by the reaction of the horseradish peroxidase (HRP) enzyme to the substrate tetramethylbenzidine (TMB). This reaction produces a blue color. A yellow color is formed after stopping the reaction with an acidic solution. The optical density is read on a standard plate reader at 450 nm. The total amount of IgG Antibody Enzyme Conjugate detected is directly proportional to the amount of Total Human IgG present in the sample.
REFERENCES & SALIVARY IGG RESEARCH
- Engstrom PE, Norhagen G, Osipova L, Helal A, Wiebe V, Brusco A, et al. Salivary IgG subclasses in individuals with and without homozygous IGHG gene deletions. Immunology. 1996;89(2):178-82.
- Madar R, Straka S, Baska T. Detection of antibodies in saliva–an effective auxiliary method in surveillance of infectious diseases. Bratisl Lek Listy. 2002;103(1):38-41.
- Brandtzaeg P. Secretory immunity with special reference to the oral cavity.Journal of oral microbiology. 2013;5.
- Hettegger P, Huber J, Passecker K, Soldo R, Kegler U, Nohammer C, et al. High similarity of IgG antibody profiles in blood and saliva opens opportunities for saliva based serology. PloS one. 2019;14(6):e0218456.
- Heaney JLJ, Phillips AC, Carroll D, Drayson MT. The utility of saliva for the assessment of anti-pneumococcal antibodies: investigation of saliva as a marker of antibody status in serum. Biomarkers. 2018;23(2):115-22.
- Brandtzaeg P. Do salivary antibodies reliably reflect both mucosal and systemic immunity? Annals of the New York Academy of Sciences. 2007;1098:288-311.
- Riis JL, Bryce CI, Stebbins JL, Granger DA. Riis, JL et al. Salivary total Immunoglobulin G as a surrogate marker of oral immune activity in salivary bioscience research. Brain Behavior and Immunity. 2020; 1(100014)
SALIMETRICS ASSAY #1-4002
The Salimetrics Salivary Total Human IgM (ImmunoglobulinM) Enzyme Immunoassay Kit was specifically designed to quantify levels of total human IgM in saliva samples and other oral fluids , which can be used for sample quality assurance and as inclusion criteria when using oral fluid as an alternative sample type for serological studies. Pathogen specific IgM is indicative of recent acute exposure, and when used together with total IgM, researchers can confidently track outbreak scenarios and minimize false negatives due to inadequate total IgM levels while using the convenience of oral fluid sampling.
Immunoglobulin M (IgM) is present in two major forms in the circulation. Natural IgM is a low-affinity pentameric molecule that binds to invading pathogens without requiring prior exposure. This form acts to directly neutralize viruses, activate complement, trigger phagocytosis, and drive antibody dependent cell mediated cytotoxicity. Adaptive IgM is the first antibody produced in response to invading pathogens and functions in a similar way to natural IgM but is a high-affinity molecule. The majority of IgM is pentameric and includes the J chain which is also a key structural component found in polymeric IgA. IgM also naturally forms hexamers, however, in this case the J chain is absent.
TECHNICAL SUMMARY
| ASSAY PROTOCOL |
| Download |
| SPECIFICATIONS | |
| Catalogue#: | 1-4002 |
| Regulatory Status: | RUO |
| Format: | 96-well plate |
| Assay Time: | ~ 3 hrs |
| Sample Volume/Test: | 10 µL |
| Sensitivity: | 0.03 ng/mL |
| Assay Range: | 0.39 – 25 ng/mL |
| Storage Requirements: | 2-8°C |
| TESTS PER KIT | |
| Singlet: | 76 |
| Duplicate: | 38 |
| TARGET ANALYTE |
| Salivary IgM |
| TECHNICAL DOCUMENTATION |
| Stop Solution SDS |
SALIVARY IGM ASSAY KIT OVERVIEW
INTENDED USE
The Salimetrics® Salivary Human Total IgM ELISA Kit is an enzyme-linked immunoassay specifically designed and validated for the quantitative measurement of human total IgM in oral fluid. It is not intended for diagnostic use. This assay kit was optimized for human salivary research and has not been validated for other human sample types, such as serum or plasma or samples from other species. For further information about this kit, its application, or the procedures in this insert, please contact the technical service team at Salimetrics or your local sales representative.
INTRODUCTION
Immunoglobulin M, or IgM, is one of five classes of antibodies found in humans, and is present as part of both the natural and adaptive immune response. Natural IgMs are low affinity pentameric molecules that bind to invading pathogens without requiring prior exposure. They act to directly neutralize viruses, activate complement, trigger phagocytosis and drive antibody dependent cell cytotoxicity. Adaptive IgM is the first antibody produced in response to invading pathogens and functions in a similar way to natural IgM, but are higher affinity molecules and are an important part of the adaptive immune response (4). IgM is the first immunoglobulin molecule found in the fetus, developing in the second half of pregnancy. The majority of IgM is pentameric and includes a molecule referred to as the J chain, a key structural component also found in polymeric IgA. IgM also forms tetramers and hexamers, however in this case the J chain is absent. In contrast to IgG, IgM antibodies peak early in the course of humoral immune responses waning after several weeks and are thus useful in the determination of recent infections or pathogen exposure. One important use for measuring antigen specific IgM in saliva is during serological surveys when assessing outbreaks of infectious diseases, for instance with the recent COVID-19 outbreak. Similar to IgM, the major source of IgM in saliva is blood, and its entry is through gingival crevicular fluid (GCF). Due to this, the total amount of IgM can vary depending on the level of serum component representation. Therefore, when measuring pathogen specific IgM, total IgM can be used to qualify a saliva sample to assure sufficient levels of total IgM in the saliva sample and provide confidence in the pathogen specific IgM results. In this regard, total IgM may be essential to prove a negative pathogen specific test result. In addition, this assay may be used to qualify samples for testing after sample storage.
SALIVARY HUMAN TOTAL IgM ASSAY PRINCIPLE
This is an indirect sandwich ELISA kit. A “sandwich” is formed when the pre-coated capture Anti-Human IgM antibody present on the plate binds IgM in standards & samples, which is then bound by the Anti-Human IgM detection antibody linked to horseradish peroxidase. After each incubation, unbound components are washed away. Bound Anti-Human IgM Antibody Enzyme Conjugate is then added and the levels measured by the reaction of the horseradish peroxidase (HRP) enzyme to the substrate tetramethylbenzidine (TMB). This reaction produces a blue color. A yellow color is formed after stopping the reaction with an acidic solution. The optical density is read on a standard plate reader at 450 nm. The total amount of IgM Antibody Enzyme Conjugate detected is directly proportional to the amount of Total Human IgM present in the sample.
REFERENCES & SALIVARY IGM RESEARCH
- Fereidan-Esfahani M, Nayfeh T, Warrington A, Howe CL, Rodriguez M. IgM Natural Autoantibodies in Physiology and the Treatment of Disease. Methods in molecular biology. 2019;1904:53-81.
- Michaud E, Mastrandrea C, Rochereau N, Paul S. Human Secretory IgM: An Elusive Player in Mucosal Immunity. Trends Immunol. 2020;41(2):141-56.
- Madar R, Straka S, Baska T. Detection of antibodies in saliva–an effective auxiliary method in surveillance of infectious diseases. Bratisl Lek Listy. 2002;103(1):38-41.
- Brandtzaeg P. Do salivary antibodies reliably reflect both mucosal and systemic immunity? Annals of the New York Academy of Sciences. 2007;1098:288-311.
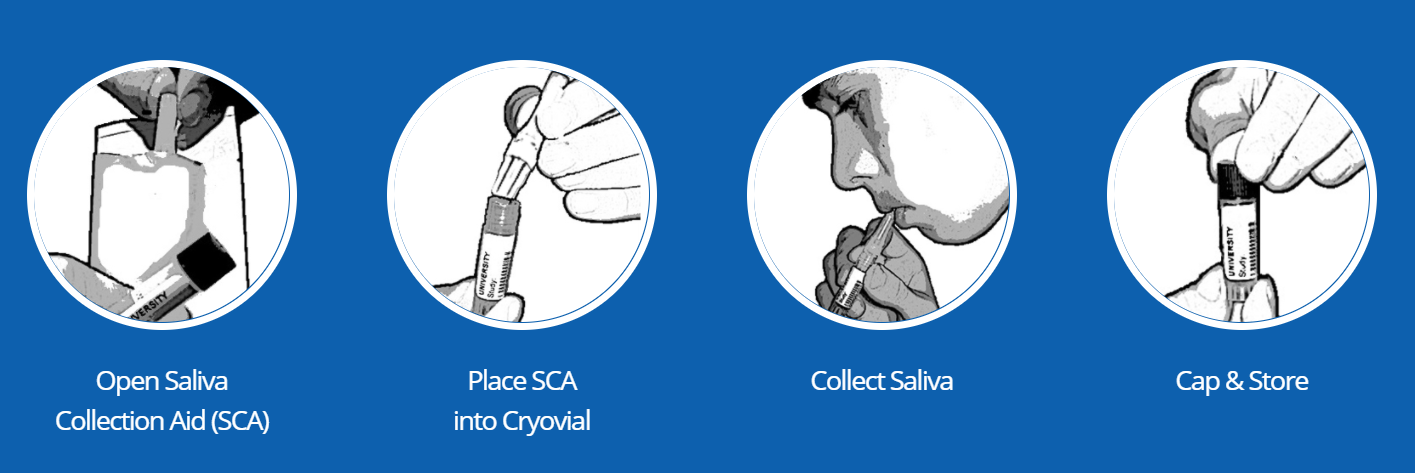
Collection Methods: Passive Drool using the Saliva Collection Aid
Introduction: Whole saliva collected by passive drool is the gold standard when collecting oral fluid for
biological testing. It avoids localized secretions of specific salivary glands providing a more consistent
specimen. Whole saliva can be easily evaluated for volume collected and for salivary flow rate. Free
from being compromised by absorbing materials used with other collection methods, whole saliva can
be assayed for all analytes of interest.
The Saliva Collection Aid (SCA) is an ideal collection tool for collecting whole saliva (passive drool). Its
ease of use reduces participant burden and improves compliance for collecting whole saliva.
The Saliva Collection Aid offers the following benefits:
✓Simple and easy to use, single use
✓ Individually packaged in a clean, foil pouch (nonsterile), (item 5016.02)
✓ Ready-to-go instructions
✓ Comfortable, no-mess collection
✓ Universal fit with external threaded cryovials
✓ Vented design reduces sample foaming
✓ Collection directly into cryovials, reducing freezer storage space
✓ Use for participants 6 years of age and older*
✓ Constructed of polypropylene
✓ Eliminates time and material needed to transfer specimen to storage vials in the lab
*NOTE: Sample collection with passive drool is designed for saliva donors who can follow simple instructions. For children younger than approximately
4 years of age, there may be wide ranging individual differences in their capability of collecting whole saliva. Thus, we encourage pilot study.
Passive Drool Cautions:
1. Use only as directed; Use each Saliva Collection Aid only once.
2. Do not use this device for children under the age of three (3), or without adult supervision.
3. Do not disassemble or pull apart device; discard if disassembled.
Materials Needed:
• Cryovials (Salimetrics Item No. 5004.01-06)
• Saliva Collection Aid (Item No. 5016.02 or 5016.02B)
• Bar-coded labels (Item No. 5009.07)
• 2” swab storage tubes boxes (Item No. 5023.05)
*NOTE: Reserve a small amount of air space in the vial to accommodate liquid expansion during freezing.
Sample Handling and Processing (As described in the Saliva Collection Handbook):
• Immediately after collection, freeze samples at or below -20°C. If freezing is not possible, refrigerate immediately at 4°C and
maintain at this temperature for no longer than necessary (ideally less than 2 hours) before freezing at or below -20°C (temperature of a regular household freezer).
• Samples stored for more than 4 months should be frozen at -80°C.
• Freeze-thaw cycles should be minimized for some analytes. It is critical that storage conditions are researched prior to initiation of sample collection.
• It is recommended that tubes be organized into cryostorage boxes (9×9 grids, 81 tubes) before storing or shipping.
How to Reference this SalivaBio Device in Your Research (Recommended)
“Whole saliva samples were collected with SalivaBio’s 2 mL cryovials and the Saliva Collection Aid (exclusively from Salimetrics,
State College, PA), a collection device specifically designed to improve volume collection and increase participant compliance,
and validated for use with salivary [Analytes].”
How to collect Passive Drool saliva samples with a Cryovial and SalivaBio’s Saliva Collection Aid.
Ready to provide your passive drool sample the easy way? In this video, Marci from Salimetrics demonstrates how to easily collect a passive drool sample using SalivaBio’s Saliva Collection Aid and standard cryovials.
SALIVA COLLECTION STEP-BY-STEP OUTLINE:
- Open foil pouch (if applicable) and remove the Saliva Collection Aid (SCA).
- Place ribbed-end of the SCA securely into a pre-labeled collection vial (see Caution 3 below).
- Allow saliva to pool in mouth. Then, with head tilted forward, gently guide saliva through the SCA into the vial. DO NOT SPIT/BLOW or otherwise force saliva into the tube. This will create foaming and bubbles.
- After the vial is filled to the required volume, remove and discard SCA. Attach cap to collection vial and tighten. Store your sample as recommended.
Passive Drool Cautions:
- Use only as directed; Use each Saliva Collection Aid only once.
- Do not use this device for children under the age of three (3), or without adult supervision
- Do not disassemble or pull apart device; discard if disassembled.
How to collect Passive Drool saliva samples with a Cryovial and SalivaBio’s Saliva Collection Aid (96 Well Format).
Ready to provide your passive drool sample the easy way? Watch this video where Marci from Salimetrics demonstrates this simple method for passive drool sample collection using SalivaBio’s Saliva Collection Aid (SCA-96W) and 96-Well high-throughput vials.
SALIVA COLLECTION STEP-BY-STEP OUTLINE:
- Obtain your Saliva Collection Aid (SCA-96W) and collection vial.
- Place ribbed-end of the SCA-96W securely into the provided collection vial (see Caution 3 below).
- Allow saliva to pool in mouth. Then, with head tilted forward, gently guide saliva through the SCA-96W into the vial. DO NOT SPIT/BLOW or otherwise force saliva into the tube. This will create foaming and bubbles.
- After the vial is filled to the required volume, remove and discard SCA-96W. Attach cap to collection vial and tighten. Store your sample as recommended.
Saliva Collection Cautions:
- Use only as directed; Use each Saliva Collection Aid only once.
- Do not use this device for children under the age of three (3), or without adult supervision
A sensitive, precise, rapidly scalable, COVID-19 (SARS-CoV-2, N) Salivary IgG ELISA Kit from Salimetrics is now available for pre-order in an effort to support serological surveillance research. This oral fluid antibody assay utilizes easy-to-collect, non-invasive saliva samples to identify prior COVID-19 exposure, enabling seroprevalence research studies to occur at a population-based level.
Today, Salimetrics announces the release of their SARS-CoV-2 Nucleocapsid Protein (N-protein) specific IgG antibody kit to support researchers’ seroprevalence studies related to the spread of the SARS-CoV-2 infection. This new kit follows the recent release of the SARS-CoV-2 (N-protein) IgG testing service at the Salimetrics SalivaLab, making this saliva-specific assay available to investigators worldwide. Researchers are encouraged to pre-order now to ensure that enough quantity is available for those with an immediate need.
Saliva as a biospecimen is not new, and Salimetrics has been actively developing sample collection and test methods specific for saliva for more than twenty years. Knowing that there are different types of oral fluid, which one is the right one for the application, and how to develop tests that are specific for the concentrations in saliva is often the key that makes the utility of saliva as a specimen successful.
Early in the pandemic, the Salimetrics team, collaborators and partners stepped in with their knowledge and experience to support the COVID-19 effort through the implementation of new tools. Now, deep into the COVID-19 pandemic, Salimetrics remains a steadfast partner. “We’ve mobilized our extensive expertise to enable researchers and public health officials to generate knowledge of COVID-19 infection/exposure history,” says Supriya Gaitonde, Ph.D., Salimetrics Senior Applications Scientist. “We are keenly aware that the practical and non-invasive nature of saliva and oral fluid collection has the potential to facilitate widespread and scalable surveillance when used in tests that have high sensitivity and specificity.”
This new development launches on the heels of the Salimetrics Salivary Human Total IgG and Salivary Human Total IgM assay kits. “These tools are designed specifically to enable health care professionals and researchers to determine general exposure history, but more importantly, to verify that saliva specimens included in surveillance programs have sufficient levels of total antibody to minimize the risk of false-negative determinations,” says Steve Granger, Ph.D., Salimetrics Chief Scientific Officer. “We pooled resources to design a SARS-CoV-2 IgG N-protein antibody assay that would be effective in facilitating scientific knowledge and supporting safe, scientific-based decision-making.” Developed in close coordination with colleagues and experts in clinical- and immune-surveillance and SARS-CoV-2 immunology, Salimetrics’ SARS-CoV-2 IgG (N-protein) ELISA Kit was designed to be practical, non-invasive, and to fill the urgent need for large-scale monitoring or seroprevalence of coronavirus infections.
This assay also fills a critical knowledge gap by simplifying a method to determine the actual spread of COVID-19 infections and provide identification and tracking for exposed-asymptomatic individuals. While the full extent of SARS-CoV-2 prevalence remains unclear, broad population-based immune testing will provide data to accurately define infection prevalence until treatments or vaccines become widely available. Several studies have indicated that saliva is a reliable tool for SARS-CoV-2 virus detection. This research has culminated into several EUAs for the diagnosis of COVID-19 by RT-PCR, using saliva as a sample type, including the high-profile test from Yale, performed in collaboration with the NBA. Their data identifies saliva to be an equivalent if not more sensitive sample type compared to nasopharyngeal swabs for virus detection. Considering that large-scale collections of saliva samples have begun in community settings including schools and universities to meet the increased demand for testing, these same samples can also be used to monitor the spread of virus exposure within these community settings by determining the percentage positive for antibodies to the virus. Since IgG in saliva originates from serum, antibody testing for the SARS-CoV-2 N-protein in saliva provides a valuable alternative over traditional blood-based serology which can even facilitate home-based sampling. “The science and technology for oral fluid-based research has advanced to the point where we can enable serosurveys to occur at a population-based level without risking the health and safety of researchers in obtaining samples,” says Granger.
The Salimetrics SARS-CoV-2 (N-protein) assay detects IgG-based antibodies related to the SARS-CoV-2 virus in a familiar 96-well, ELISA format, enabling researchers to screen a large number of participants for indications of past or recent infection. The SARS-CoV-2 (N-protein) Salivary IgG Assay Kit has a sensitivity of 92%, while equally maintaining a 97% specificity. While N-protein IgG remains the most dominant immunoglobulin for SARS-CoV-2 detection, Salimetrics is also developing an oral fluid-based assay for Spike protein antibodies. The release of this complementary assay kit will follow later this year.
Since 1998, Salimetrics has provided tools to the salivary bioscience community to help solve key issues related to the collection and assay of saliva and oral fluid samples. The mission at Salimetrics has always been to adapt and respond to the scientific community’s needs, provide the best tools, and ensure that each product has the highest level of utility, precision, or reproducibility. For more information, or to pre-order the COVID-19 (SARS-CoV-2, N) Salivary IgG ELISA Kit, researchers can Ask an Expert.
About Salimetrics:
Salimetrics’ assay kits and CLIA-certified testing services are used to measure salivary analytes related to stress, behaviour and development, inflammation, sleep, reproduction, health and immune function. Founded in 1998 by Douglas A. Granger, Ph.D., Salimetrics, LLC support CROs, pharmaceuticals, academic researchers and the immunodiagnostic industry around the world with innovative immunoassay products, non-invasive saliva collection methods, and laboratory testing services.
