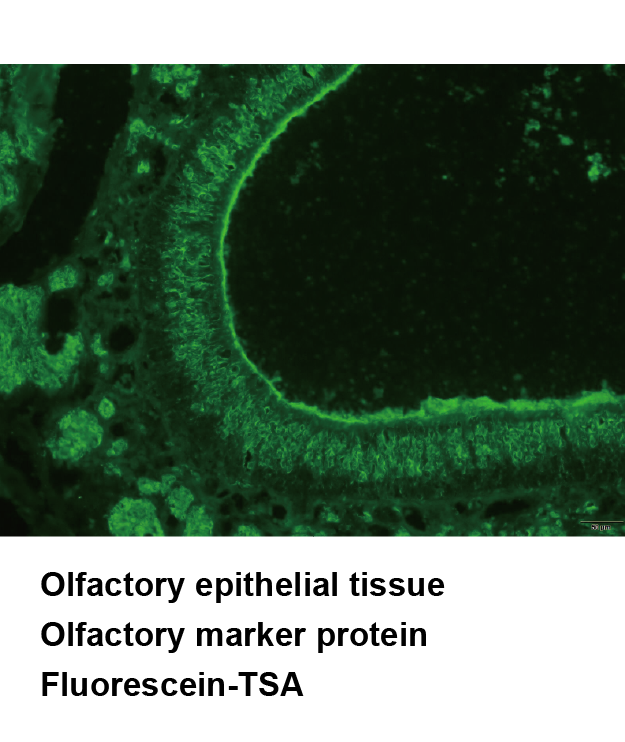
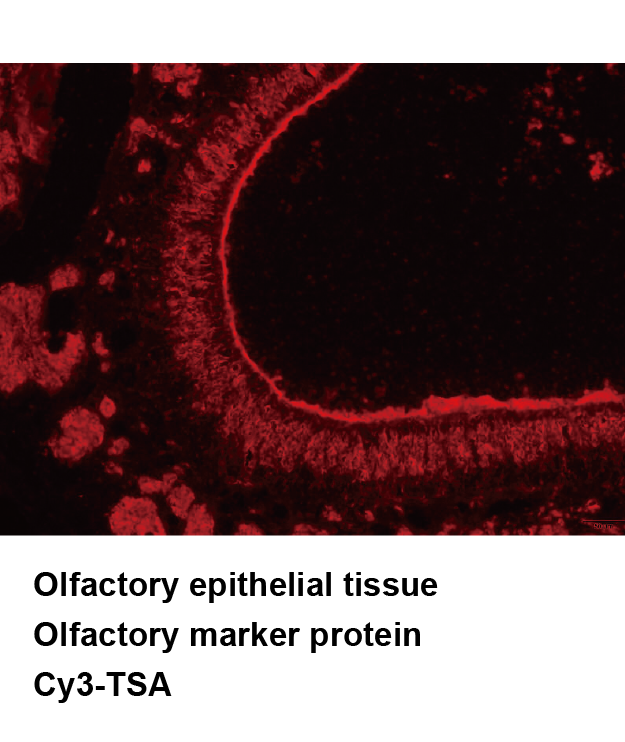
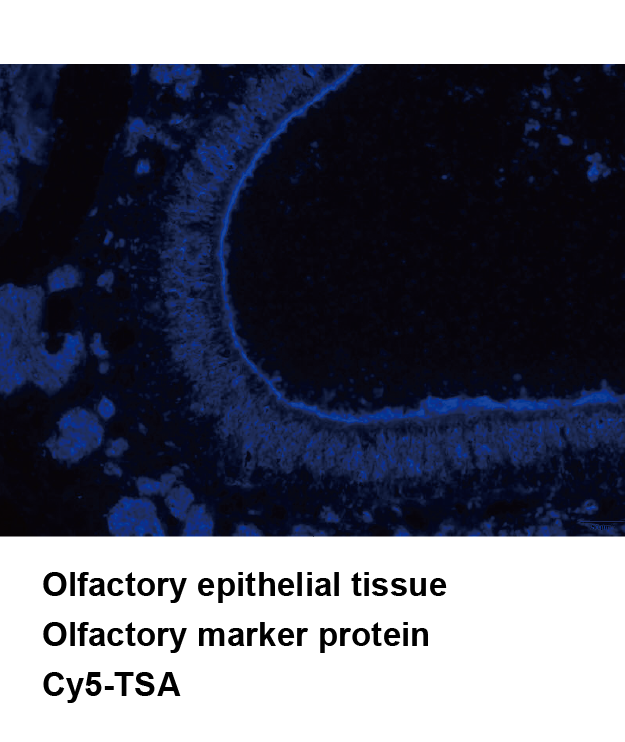
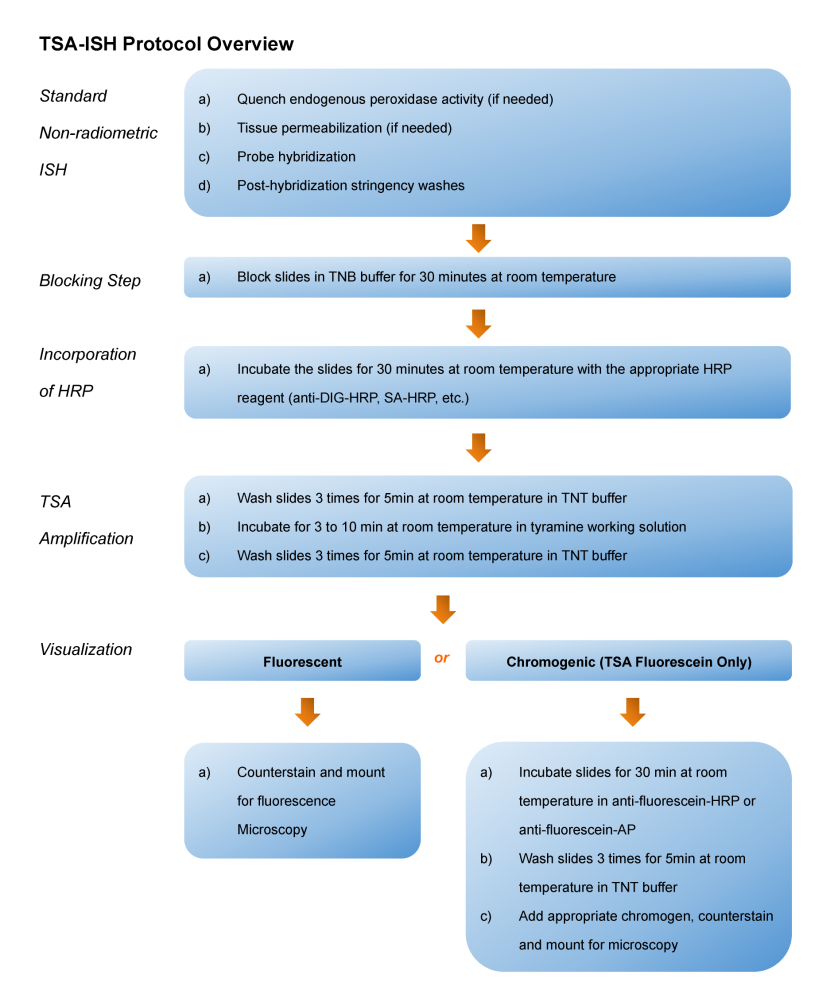
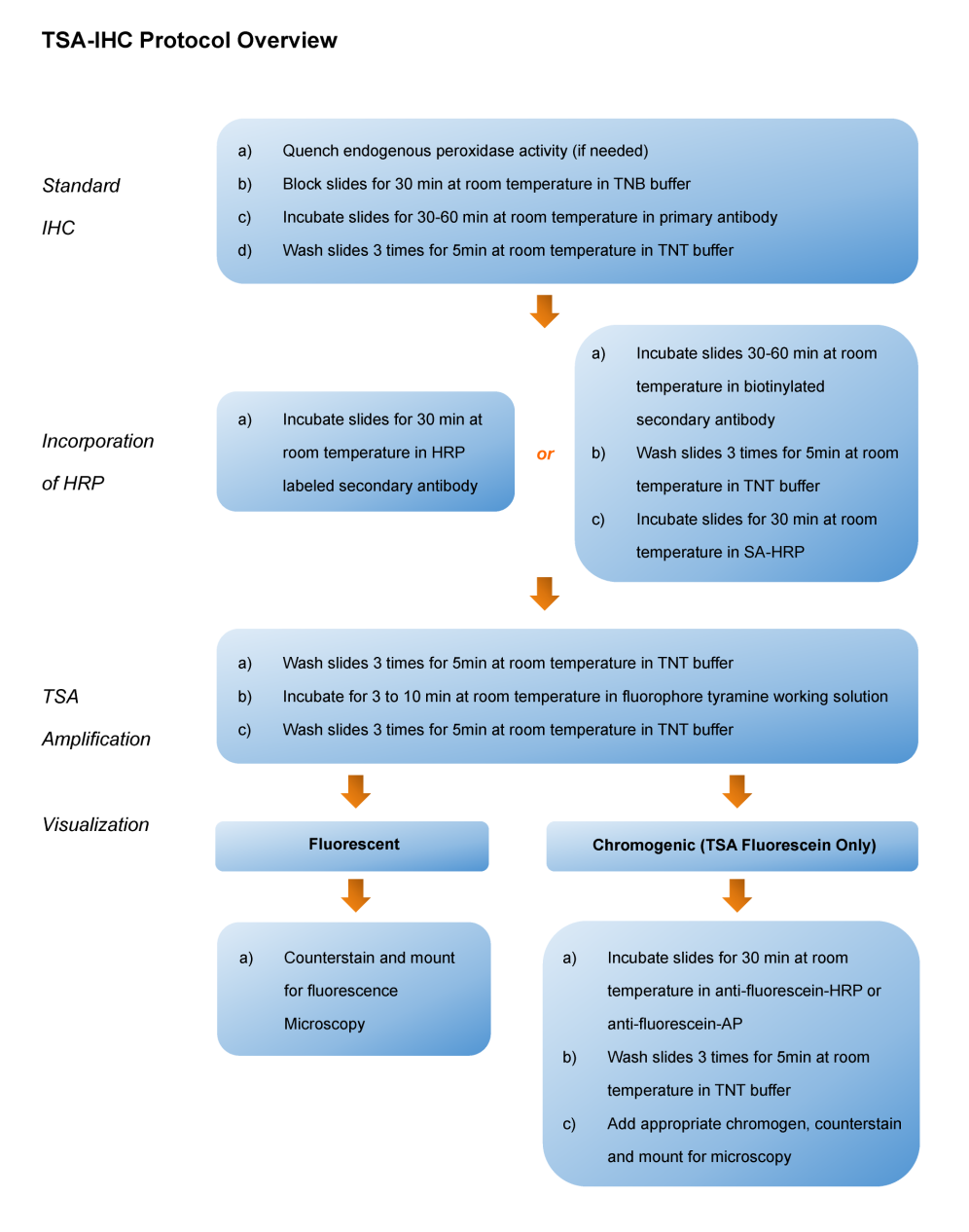
Tyramide Signal Amplification (TSA), sometimes called Catalyzed Reporter Deposition (CARD), is a highly sensitive method enabling the detection of low-abundance proteins or nucleic acid sequences in-situ, where tyramide, a phenolic compound, has the ability to bind to the electron rich surface of targets.
TSA involves horseradish peroxidase (HRP)-catalyzed deposition of tyramide on and near a target protein or nucleic acid sequence in situ. In the presence of low concentrations of H2O2, HRP is able to convert a labeled tyramide substrate into a highly reactive form that can covalently bind to tyrosine residues on proteins at or near the HRP. This generates high density tyramide labeling and is the reason for the exceptional sensitivity of this system. Tyramide can be labeled with a fluorophore or a hapten (such as biotin). The biotin on the bound tyramine serves as a tracer molecule that can be visualized using standard techniques that use avidin-biotin-enzyme complex formation reactions. The conjugation of tyramine molecules to a hapten or fluorochrome make the indirect and direct fluorescence detection of enzymatically deposited tyramides possible.
TSA can increase detection sensitivity up to 100-fold. In addition, TSA can be combined with several other technologies such as nucleic acid labeling, primary and secondary antibodies, avidin and lectin conjugates, cytoskeletal stains, organelle probes and cell tracers based detection techniques. TSA can be used to improve current immunohistochemistry (IHC), immunofluorescence (IF), or in-situ hybridization (ISH), based protocols using existing imaging hardware, for example microscopes.