Nanobodies were first reported by Belgian scientists Hamers et al. in Nature in 1993. A naturally occurring light chain-deficient antibody exists in alpaca peripheral blood, which contains only one heavy chain variable region and two conventional CH2 and CH3 regions but does not readily adhere to each other or even aggregate into blocks as artificially modified single chain antibody fragments (scFv) do. Interestingly, the individually cloned and expressed heavy chain variable region has structural stability and antigen-binding activity comparable to that of the original heavy chain antibody and is the smallest known unit that binds the target antigen. Compared to conventional antibodies, Nanobodies have a small molecular weight, simple structure, easy genetic modification, small size, high antigen specificity, and enhanced tissue penetration and stability. These features make them an attractive option for disease diagnosis and treatment.
What should you know about Nanobodies?
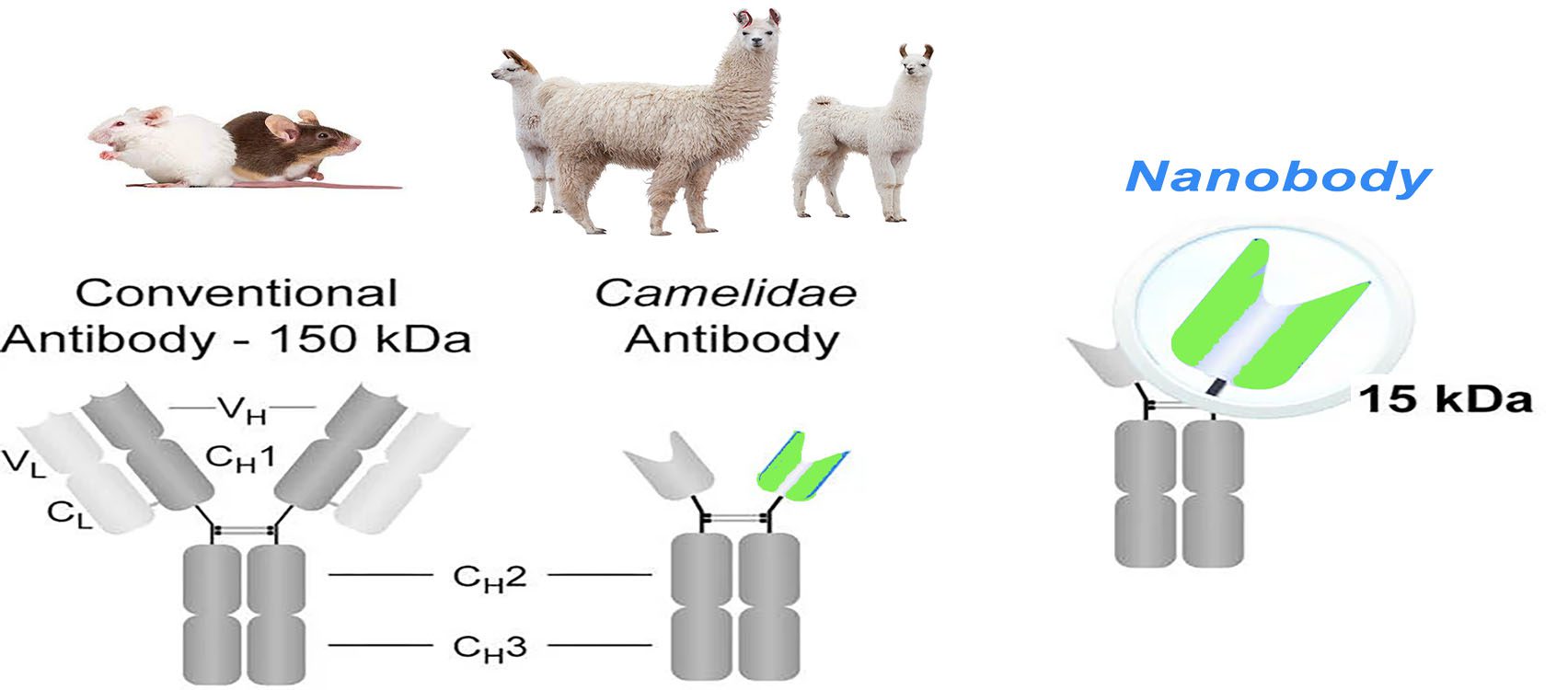
Structure Comparison
The basic structure of a normal immunoglobulin is a tetrameric polypeptide with a molecular weight of 150 kDa, consisting of two pairs of identical heavy (50 kDa) and light (25 kDa) chain polypeptides linked by disulfide bonds between the chains. The light chain consists of a variable region (VL) at the N-terminal end and a conserved region (CL) at the C-terminal end. The heavy chain consists of four or five structural domains: a variable region at the N-terminus (VH), followed by three to four constant regions (CH1, CH2, CH3, possibly CH4). These regions form a Y-shaped structure, with two antigen-binding regions sequestered by a flexible hinge region between CH1 and CH2. CH2 is part of the FC region, responsible for the antibody-dependent cytotoxic (ADCC) and complement-dependent cytotoxic effects (CDC). This Y-shaped structure is relatively conserved in mammals. At the N-terminal of the antibody, the paired VH and VL form the antigen-binding site, each containing three complementarity-determining regions (CDR1, CDR2, and CDR3) and four framework sequences that stabilize the loop structure. The heavy chain CDR3 is the most variable region in length and amino acid composition and, thus, the most unpredictable in structure.
The immune response of infected or immunized camelids produces conventional and heavy chain only antibodies, both of which have the ability to bind to antigens. Heavy chain antibodies lack the CH1 region, and CDR1 and CDR3 are longer to compensate for the reduced antigen-binding caused by the absence of the light chain. Nanobodies, on the other hand, consist of the heavy chain variable region only, and five amino acids conserved in conventional VH were changed: Leu12Ser (i.e., position 12 is often Leu in VH, while it is often Ser in nanobody), Val42Phe/Tyr, Gly49Glu, Leu50Arg/Cys, and Trp52Gly. These four sites in VH (42, 49, 50, and 52) are mainly involved in the interaction with VL, which are primarily hydrophobic amino acids but become hydrophilic in nanobodies. This sequence change increases the nanoantibody’s water solubility, reduces the nanoantibody’s aggregation, and directly leads to the inability of the nanoantibody to bind the light chain.
Advantages of Nanobodies
Nanobodies are superior to conventional antibodies in many aspects. The unique structure of single-domain antibodies perfectly overcomes the drawbacks of conventional antibodies, such as long development periods, low stability, and harsh storage conditions, making nanobodies become an emerging force in the new generation of therapeutic biopharmaceuticals and clinical diagnostic reagents. Compared with conventional antibodies, the advantages of nanobodies are:
- small molecular weight, which can penetrate the blood-brain barrier.
- high expression in prokaryotic or eukaryotic systems.
- high specificity and high affinity.
- weak immunogenicity in humans.
- good stability.
- high solubility and good absorption.
- simple humanization.
- easy to couple other molecules.
