Amphotericin B+dPEG®: Water-Soluble, Less Toxic, Potent
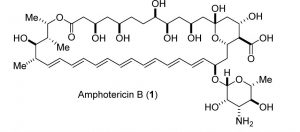
Figure 1: Structure of Amphotericin B. Image used by permission from J. Med. Chem. (2016), 59, 1197-1206, copyright 2016, American Chemical Society.
Amphotericin B (Figure 1) is the “gold standard” treatment for systemic fungal infections and diseases caused by the parasite Leishmania. Sometimes it is the only effective treatment because drug resistance renders other treatments useless. Systemic fungal infections are an increasingly serious, widespread problem in medicine. Patients with weakened or suppressed immune systems (caused by HIV/AIDS, diabetes, organ transplants, some cancer treatments) are especially at risk. An estimated 1.5-2 million people die each year from systemic fungal infections (1). Despite its “gold standard” label, Amphotericin B has several well-known difficulties.
A polyene macrolide antifungal drug, Amphotericin B was first discovered in 1953 from the fermentation culture of the bacterium Streptomyces nodosus. It entered the pharmaceutical market in 1958 (2)(3)(4). Nearly insoluble in water, the drug is not absorbed from the human gastrointestinal tract and must be administered intravenously. Amphotericin B was first formulated as a suspension in sodium deoxycholate solution called Fungizone-Squibb (2)(7). Novel, less toxic lipid formulations have been developed recently (5)(6)(7), but these formulations cost more and require high doses to be effective, thus limiting their use (4)(7).
This drug is also toxic. Its side effects are well known, serious, and sometimes fatal (7)(8). Common side effects from taking this drug include high fever coupled with shaking chills (“shake and bake”), headache, kidney problems, low blood pressure, nausea, vomiting, unusual bruising or bleeding, and irregular heartbeat. Anaphylactic shock sometimes occurs with this drug. A 1997 report from the Center for Drug Evaluation and Research, a division of the United States Food and Drug Administration, stated that the toxicity of Amphotericin B was 1.5 mg/Kg in rats. Other reports reach similar conclusions (4)(5)(6)(7)(8).
Since its discovery, numerous researchers have attempted to modify the properties of Amphotericin B to make it more water-soluble, less toxic, or both. PEGylation is one obvious strategy for improving the water solubility of a water-insoluble drug. Greenwald, et al. (9), and Sedlak, et al. (10)(11) created PEGylated prodrug versions of the drug, but these constructs used large, dispersed, polymeric PEGs that are heterogeneous in size and, hence, difficult to characterize.
A Different Approach to Water-Soluble Amphotericin B
In a paper published in January 2016 in the Journal of Medicinal Chemistry, Assaf Halperin, Yana Shadkchan, Evgeni Pisarevsky, Alex Szpilman, Hani Sandovsky, Nir Osherov, and Itai Benhar, from Tel-Aviv University and the Israel Institute of Technology chose to work with short PEG chains of defined length and molecular weight. Specifically, they chose to create a stable amide bond on the mycosamine ring and expose a free amine at the opposite end of the linker. After testing PEG linkers with 4, 8, and 44 ethylene oxide units, they settled on the PEG8 linker, because it offered significant improvement in water solubility with little loss in efficacy. By contrast, the PEG4 linker improved water solubility, but less well than the PEG8 and PEG44 linkers. The PEG44 linker improved water solubility greatly, but the derivative’s efficacy dropped thirty-fold compared to the parent drug.
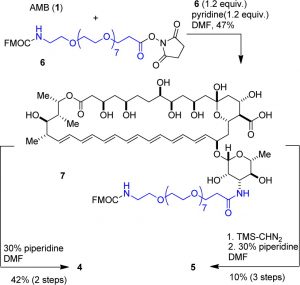
Figure 2: Synthetic scheme used by Halperin, et al., to create the two PEGylated derivatives of Amphotericin B. Image used by permission from J. Med. Chem. (2016), 59, 1197-1206, copyright 2016, American Chemical Society.
For the construction of two different PEGylated derivatives, the research group used Quanta BioDesign’s Fmoc-N-amido-dPEG®8-NHS ester, product number 10995, purchased from our German distributor, Iris Biotech, GmbH. (Click here for a complete list of our distributors.) The authors stated the reason for their choice in this way:
This approach creates a well-defined medium-molecular-weight product that can be easily separated and characterized by NMR and other common techniques …. The short and specific synthesis and well-defined structural nature of the antifungal conjugate is expected to be an advantage in clinical approval as well. (4, page 1201).
After the dPEG® was conjugated to Amphotericin B, the Fmoc protecting group was removed, exposing the free amine on the dPEG® linker. The synthetic scheme used by the authors is shown in Figure 2.
Amphotericin B-dPEG®8 Derivatives Show Promise as Clinical Replacements for the Parent Compound
In testing, both dPEG®ylated derivatives (AB1, the free acid; AM2, the methyl ester; see Figure 3) increased dramatically in water solubility, decreased considerably in toxicity, and maintained (with only a moderate decrease) efficacy.
The solubility of Amphotericin B in water is less than 1 µg/mL (<0.001 mg/mL). The AM2 derivative’s water solubility increased to 700 µg/mL (0.7 mg/mL), while AB1 increased to 5,500 µg/mL (5.5 mg/mL). Previous efforts to make useful PEGylated derivatives of Amphotericin B used carbamate linkages between the drug and the PEG. These linkages hydrolyzed readily in phosphate-buffered saline (PBS). By contrast, neither AB1 nor AM2 showed any hydrolysis in PBS after 4 hours, and both hydrolyzed less than 1% after 24 hours in PBS, demonstrating that they are stable. Furthermore, pharmacokinetic studies of AM2 in mice showed no free Amphotericin B after 24 hours, indicating that the derivatives are stable.
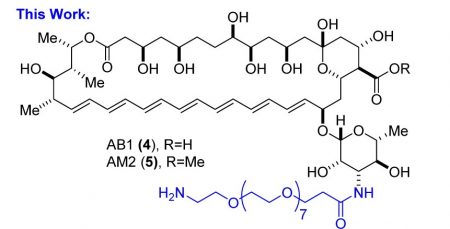
Figure 3: Structures of the dPEG®8-amine derivatives of Amphotericin B. Image used by permission from J. Med. Chem. (2016), 59, 1197-1206, copyright 2016, American Chemical Society.
The authors tested the toxicity of AB1 and AM2 against the parent drug in three ways. First, they evaluated nonspecific toxicity of all three compounds by suspending fresh human red blood cells (hRBCs) in PBS and measuring lysis of the cells (hemolysis) in the presence of Amphotericin B or the derivatives. Second, they assayed the viability of mouse embryonic fibroblast cells using an MTT assay (for a detailed, technical discussion of the MTT assay plus procedures for conducting the assay, click here). Third, they measured the LD50 of the three compounds by intravenous injection in mice.
In the first test, Amphotericin B was highly toxic as expected. AB1 was less toxic to hRBCs than the parent compound, while AM2 caused very little hemolysis and thus demonstrated little non-specific toxicity.
The MTT assay (see Figure 4) showed dramatic differences between Amphotericin B and the derivatives. The MTT assay measures the half-maximal inhibitory concentration, also known as the IC50 (see also here), of a compound against cells in culture. In the assay, AB1 was forty times less toxic to the mouse embryonic fibroblasts than Amphotericin B, while AM2 was at least 600 times less toxic.
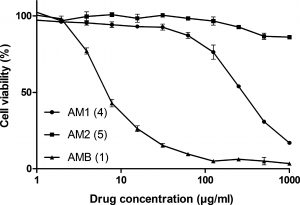
Figure 4: MTT Assay comparing Amphotericin B to the AB1 and AM2 derivatives. A color version of this image is at the beginning of this post. AM1 (should be AB1) and AM2 are the derivatives shown in Figure 3. AMB is Amphotericin B (see Figure 1 for structure). Image used by permission from J. Med. Chem. (2016), 59, 1197-1206, copyright 2016, American Chemical Society.
The in vivo toxicity testing showed dramatically different results among Amphotericin B, AB1, and AM2. The LD50 of Amphotericin B was 1.1 mg/Kg. Mice injected with twice the LD50 died instantly. By contrast, AB1 was not toxic to mice at the maximum injected dose of 22 mg/Kg, and AM2 was not toxic to mice at the maximum injected dose of 42 mg/Kg.
Efficacy (a measure of the potency of antifungal activity) was assessed both in vitro and in vivo. Both AB1 and AM2 retained activity against a broad spectrum of fungi but were 2-16 times less potent than Amphotericin B. When the length of time needed to kill all fungal cells was measured, AM2 eliminated all fungi in vitro within 2 hours, similar to Amphotericin B. In in vivo testing, Amphotericin B at 1 mg/Kg (near the LD50) cured 10 of 12 mice of fungal infection, compared to AM2, which achieved the same result using 3.5 mg/Kg. AM2, though, cured all mice of fungal infections at a dose of 7 mg/Kg, something that non-PEGylated parent drug could not do, because it was too toxic.
Interestingly, AM2 had a shorter serum half-life than Amphotericin B. This result seems odd at first glance because PEGylation is one of the primary means by which the serum half-life of therapeutic agents is extended. The reason for the difference is that Amphotericin B binds serum proteins, which lengthens the drug’s serum half-life. Testing revealed that AM2 does not bind strongly to serum proteins. This allows it to be removed much more quickly from the body.
AB1 and AM2 both show promise as clinical antifungal agents. The increased water solubility and lower toxicity compared to Amphotericin B are important, but it is also important that the efficacy of the derivatives is not greatly diminished from the parent compound. The discrete PEGylation (dPEG®) reagent simplifies analysis of the products, and the specific dPEG® reagent chosen, PN10995, is ideal for conjugating the Amphotericin B derivatives to other molecules after removal of the Fmoc group protecting the terminal amine.
This work also highlights the remarkable power of a relatively small dPEG® linker. Many research groups tend to think that large, dispersed PEGs are the solution to their water solubility problems. This research shows that a dPEG®8 linker imparts significant water solubility and reduces toxicity, with only a modest impact on efficacy. This is truly important for understanding the power and ability of discrete PEGylation.
References
- Denning, D. W.; Bromley, M. J. Infectious disease: how to bolster the antifungal pipeline. Science (27 March 2015), 347(6229). 1414-1416.
- Dutcher, James D. The discovery and development of Amphotericin B. Dis. Chest (October 1968), 54(supplement 1), 40-42.
- Gallis, Harry A.; Drew, Richard H.; and Pickard, William W. Amphotericin B: 30 years of clinical experience. Clin. Infect. Dis. (1990), 12(2), 308-329.
- Halperin, Assaf; Shadkchan, Yana; Pisarevsky, Evgeni; Szpilman, Alex M.; Sandovsky, Hani; Osherov, Nir; and Benhar, Itai. Novel water-soluble Amphotericin B-PEG conjugates with low toxicity and potent in vivo efficacy. J. Med. Chem. (2016), 59, 1197-1206.
- Larabi, M.; Pages, N.; Pons, F.; Appel, M.; Gulik, A.; Schlatter, J.; Bouvet, S.; and Barrat, G. Study of the toxicity of a new lipid complex formulation of amphotericin B. J. Antimicrob. Chemother. (2004), 53, 81-88.
- Souza, L. C.; Campa, A. Pharmacological parameters of intravenously administered amphotericin B in rats: comparison of the conventional formulation with amphotericin B associated with a triglyceride-rich emulsion. J. Antimicrob. Chemother. (July 1999) 44(1): 77-84.
- Hamill, Richard J. Amphotericin B Formulations: A Comparative Review of Efficacy and Toxicity. Drugs (2013), 73: 919-934
- Laniado-Laborín, Rafael; and Cabrales-Vargas, Maria Noemí. Amphotericin B: side effects and toxicity. Rev Iberoam Micol. (2009) 26(4):223–227.
