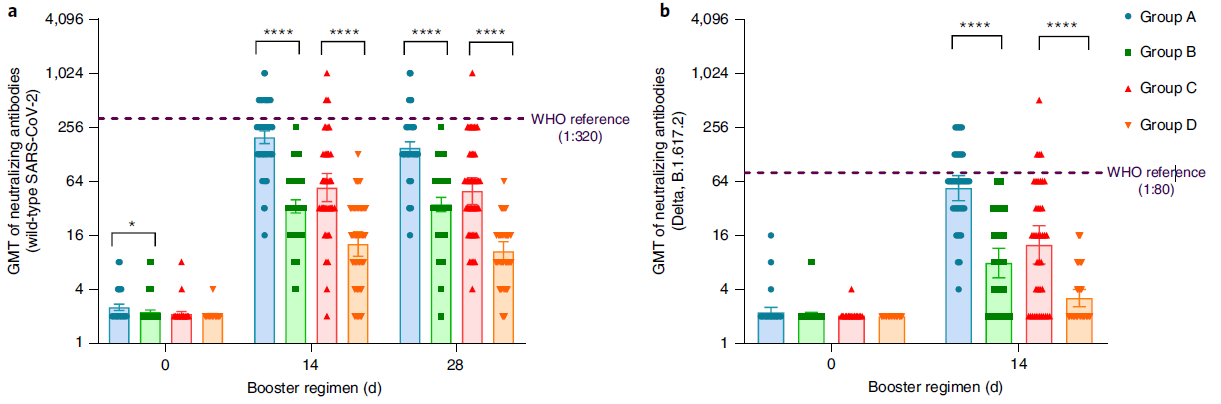
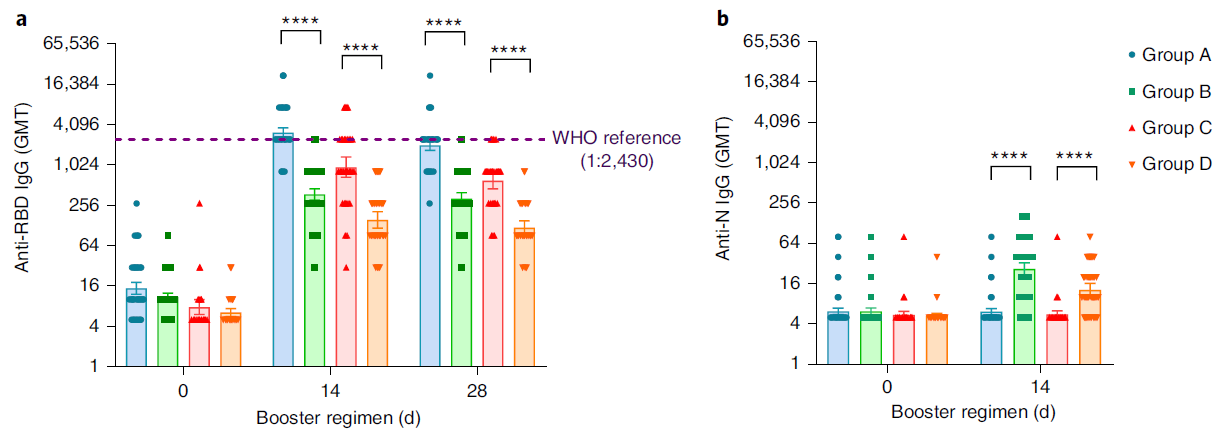
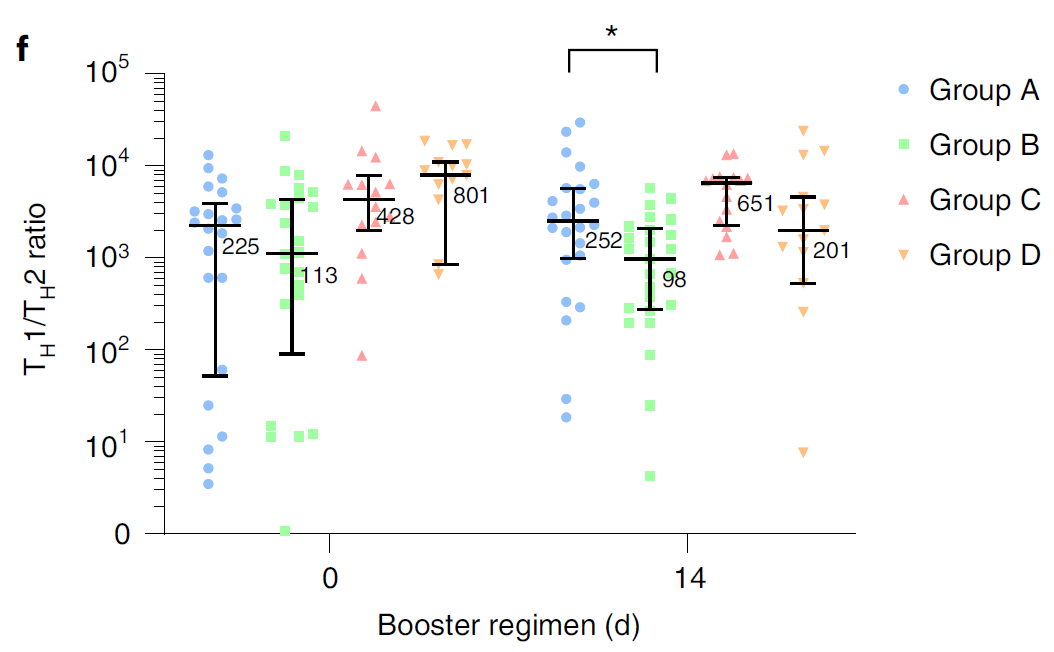
At present, more than 2 billion doses of the COVID-19 vaccine have been administered in mainland China, of which the vaccination rate of CoronaVac is more than 50%1. With the continuous emergence of SARS-CoV-2 variants and the weakening of neutralizing antibodies from inactivated vaccines, the protective effect of vaccination gradually decreases2. The schedules of Sequential vaccination that integrate COVID-19 vaccines across different platforms may promote antibody affinity maturation and affect the breadth of vaccine-elicited neutralizing antibodies3.
On January 27, 2022, Professor Zhu Fengcai and Academician Chen Wei’s team published an important article in the journal Nature Medicine. The article confirmed that the level of humoral immunity induced by sequential booster vaccination with recombinant adenovirus type 5 (AD5) vaccine (hereinafter referred to as ‘intramuscular injection of Convidecia®’) was higher than CoronaVac homologous vaccination, and the safety was good. The intramuscular injection of Convidecia® used is produced by the team of Academician Chen Wei and CanSino Bio Co., Ltd. (hereinafter referred to as ‘CanSino Bio’, CanSino-U 688185. SH, CanSino-B 06185. HK) jointly developed.